Abstract
Objective
To characterize the temporal pattern of a panel of blood and urine biomarkers in an animal model of fecal peritonitis and recovery
Design
Prospective observational animal study
Setting
University research laboratory
Subjects
Male Wistar rats
Interventions
A fluid-resuscitated, long-term (3 day) rat model of sepsis (fecal peritonitis) and recovery was used to understand the temporal association of sepsis biomarkers in relation to systemic hemodynamics, inflammation, and renal function. At pre-defined time points (3, 6, 12, 24, 48, 72h), animals (≥6 per group) underwent echocardiography, blood and urine sampling, and had kidneys taken for histological analysis. Comparison was made against sham-operated controls and naïve animals.
Measurements and main results
The systemic pro-inflammatory response was maximal at 6 hours, corresponding with the nadir of stroke volume. Serum creatinine peaked late (24h), when clinical recovery was imminent. Histological evidence of tubular injury and cell death was minimal. After a recovery period, all biomarkers returned to levels approaching those observed in sham animals. Apart from urine clusterin and IL-18, all other urinary biomarkers were elevated at earlier time-points compared to serum creatinine. Urine NGAL was the most sensitive marker among those studied, rising from 3h. While serum creatinine fell at 12h, serum cystatin C increased, suggestive of decreased creatinine production.
Conclusions
Novel information is reported on the temporal profile of a panel of biomarkers in sepsis in the context of systemic and renal inflammation and recovery. Insight into the pathophysiology of AKI is gleaned from the temporal change markers of renal injury (urine NGAL, KIM-1, calbindin), followed by a marker of cell cycle arrest (urine IGFBP7) and, finally, by functional markers of filtration (serum creatinine and cystatin C). These clinically relevant findings should have significant influence on future clinical testing.
Keywords: Biomarkers, Sepsis, Acute kidney injury, Animal model
Introduction
Sepsis is the underlying cause in half the cases of acute kidney injury (AKI) (1). Up to 5% of total ICU admissions require renal replacement therapy for AKI and a third of them die (1, 2). Despite the clinical impact of septic AKI management is limited to supportive care. The current lack of a sensitive marker of early parenchymal kidney injury reduces the window of opportunity for early effective intervention to prevent renal dysfunction and failure. Numerous blood and urine markers of renal injury/dysfunction are being promoted but, at present, remain poorly characterized. Few studies have investigated the utility of biomarkers in predicting recovery from AKI (3). A complementary panel of markers will likely enhance interpretation of this dynamic process, although studies in septic patients are confounded by an inability to precisely time the onset of sepsis. Thus, the temporal relationship of these biomarkers to the onset, progression and recovery of renal dysfunction and injury is unknown. Serial measurements of a panel of renal biomarkers in a well-characterized animal model with a defined onset of polymicrobial sepsis followed by recovery will provide invaluable information translatable to the patient.
Animal models of sepsis offer the advantage of knowing precisely when ‘time zero’ occurs and also allow control of volume status and other conditions in a relatively homogenous population. However, to be representative of the human condition, they must simulate many of the physiological and pathological aspects of sepsis, including a proper infectious insult, an adequate duration of study, and fluid resuscitation to avoid the consequences of untreated hypovolemia leading to organ hypoperfusion. We used a clinically relevant and well-characterized model of sepsis and recovery to define parallel changes in global hemodynamics, biochemistry, renal histology, serum cytokines, and a panel of biomarkers (4, 5).
Materials and Methods
In vivo experiments
Male Wistar rats (Charles River, Margate, Kent, UK) weighing 300-375g were used. Experiments were performed under a Home Office Project Licence (PPL 70/7029) and local UCL Ethics Committee approval. The rats were housed in cages of six on a 12-12 h light–dark cycle. Six time-points were selected to represent early (3, 6, 12h), established (24h) and recovery (48 and 72h) phases of sepsis.
All invasive and imaging techniques were performed under a brief period of isoflurane anesthesia with the animal breathing spontaneously, as described previously (4, 5). Following tunneled internal jugular line placement, rats were placed in individual cages mounted on the tether/swivel system to secure the intravenous catheter and allow unimpeded movement with free access to food and water. The reasons for tunneled CVC insertion are two-fold; firstly to prevent the line from being pulled out by the animal and thus enable ongoing fluid resuscitation, and secondly to reduce the risk of CVC-related infection. At 24h post-instrumentation, sepsis was induced by intraperitoneal injection of fecal slurry (Please see Appendix for more detail). This was not performed in sham animals to prevent inadvertent bowel perforation.
Antibiotics were not administered to avoid any confounding drug-induced nephrotoxicity. The optimal volume and rate of fluid administration (with 1:1 mix of 5% glucose and Hartmann’s solution) to maintain intravascular volume based on echo parameters has been previously determined (4). Sham animals received a similar fluid regimen.
Echocardiography was performed prior to each fluid bolus, and at the terminal time-point as previously described (4, 5). At the terminal time-point, a carotid arterial line was inserted under isoflurane anesthesia with the animals breathing spontaneously. Blood gas analysis was performed using 0.2 ml arterial blood taken into heparinized capillary tubes (ABL-70 analyzer, Radiometer, Copenhagen, Denmark).
A laparotomy incision was made and a 22-gauge needle used to aspirate urine via bladder puncture. The left kidney was isolated and the upper pole placed into formalin and the rest snap-frozen in liquid nitrogen. Cardiac puncture was then performed to obtain blood which was placed in a heparinized tube and centrifuged at 6500 rpm for 10 min. The serum was siphoned off, aliquots taken, snap-frozen in liquid nitrogen, and stored at -80°C.
Serum and tissue sample measurements
DuoSet ELISA kits (R&D Systems, Minneapolis, MN, and BD Biosciences, Oxford, Oxon, UK were used to assess serum cytokine levels according to the manufacturers’ instructions. Absorbance was read at 450 nm using a spectrophotometric ELISA plate reader (Anthos HTII; Anthos Labtec, Salzburg, Austria).
MILLIPLEX®MAP multi-analyte panels (Merck Millipore, Watford, Herts, UK) were used for simultaneous detection and quantification of eight biomarkers and cytokines/chemokine in rat urine, including NGAL, cystatin C, IL-18, MCP-1, clusterin, calbindin, osteopontin, and KIM-1. The same technique was used to determine serum levels of IL-18. Assays were performed according to the manufacturer’s protocols. The plate was read on a Bio-Plex 200 multiplex system (Bio-Rad, Hemel Hempstead, Herts, UK). Urine TIMP-2 and IGFBP7 were analysed by ELISA as described previously (6). Renal function (serum creatinine) was analyzed using the Jaffe assay by the Clinical Pathology laboratory at the Royal Free Hospital, London, UK. Where urine biomarkers were analyzed, matched creatinine values were used.
Immunohistochemistry
Kidneys were fixed for 24-72 hours in formalin, transferred to 70% ethanol, and embedded in paraffin. Sections were then cut into 5 μm slices and mounted onto glass slides. For all histological analyses, sections were examined using an Olympus BX4 microscope (Olympus Optical, London, UK) at x20 magnification. For assessment of tubular injury (tubular dilatation, brush border loss, and tubular cast formation), sections were stained with Periodic acid-Schiff (PAS). Ten random fields of view of the cortex were analyzed for each section at x20 magnification under a light microscope. Apoptosis was identified by DNA fragments in situ using the terminal deoxyribonucleotidyl transferse (TdT)-mediated biotin-16-dUTP nick-end labeling (TUNEL assay) using the TACS® TdT In Situ Apoptosis Detection Kit (R&D Systems). The total number of apoptotic bodies per x20 field were counted manually and an average taken for each group. A total of three slides for the septic groups (with at least one from the poor prognosis subgroup) and two from the naïve groups from time-points 6, 12, 24, 48, and 72h were selected.
Statistics
Analyses were performed using SPSS (Version 22, IBM) and graphs drawn using Graphpad Prism Version 5.0d (GraphPad Software Inc. La Jolla, CA, USA). Continuous variables are presented as median (interquartile range). Differences in continuous variables between groups were compared using Mann Whitney U test. Pearson’s correlation was performed to assess the degree of correlation between serum and urine levels of biomarkers (cystatin C, NGAL, IL-18, and MCP-1). A p-value <0.05 was taken as statistically significant.
Results
Baseline measurements (weight, temperature, HR, SV, CO) were similar between groups. In total, 19 animals died (20%, as per the severity of the model). All deaths occurred after the 12h time point and before the 48h time point. These animals were not used for biomarker/biochemistry analyses. The sampling time (involving sacrifice of the animals) was randomized. All animals intended for sampling at early time points survived, whereas some of the animals intended for sampling at a late time point succumbed prior to reaching this time point.
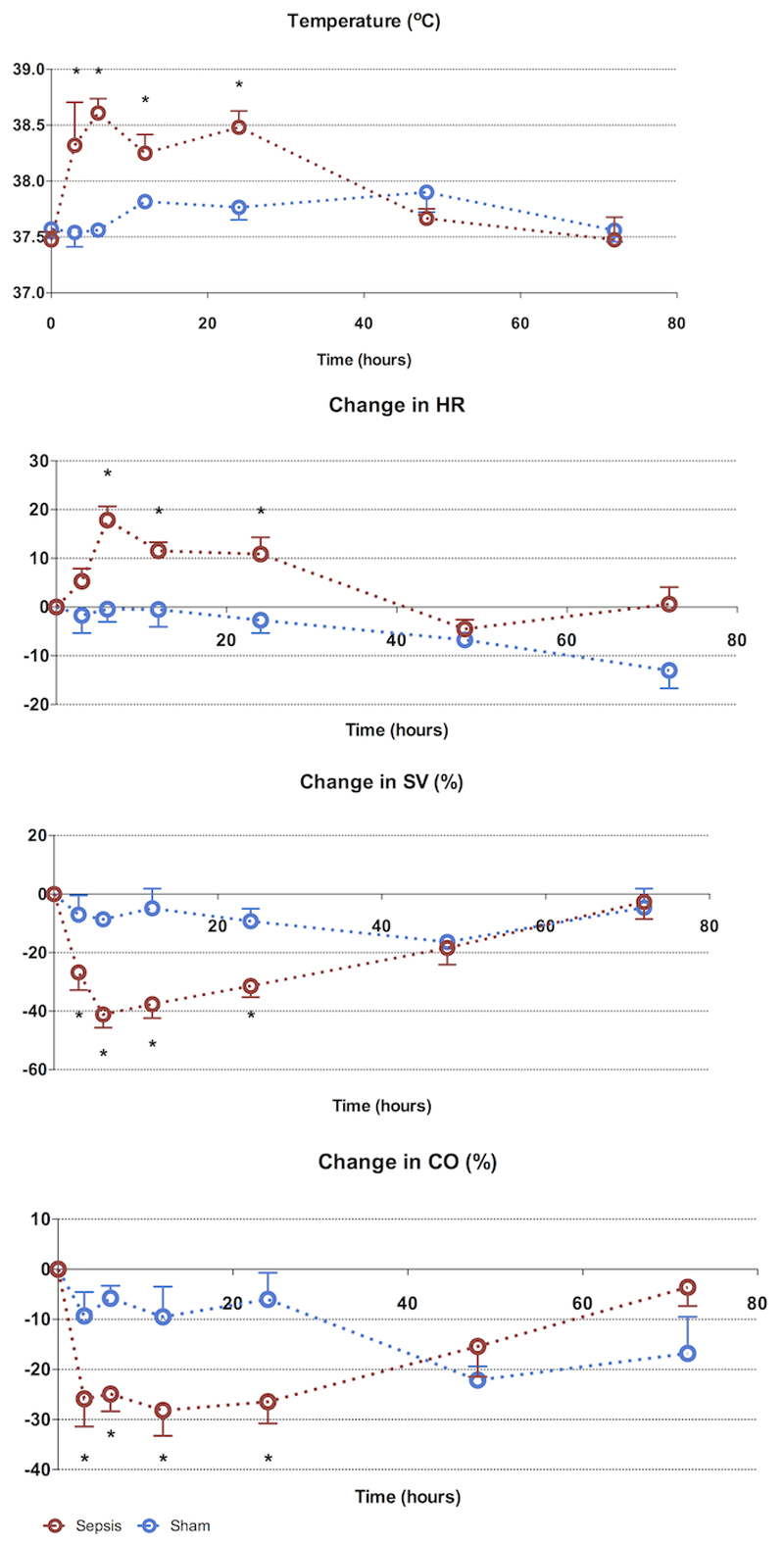
Physiological markers (Figure 1)
Figure 1. 72-hour characterization of sepsis and recovery phases - systemic variables.
Septic animals develop a tachycardia, fall in stroke volume and fever early (3-6h), which resolves from 24h to reach baseline values at 72h. Points and whiskers represent median and interquartile range respectively. * p<0.05 sham vs sepsis; (*): p=0.05-0.06 sham vs sepsis)
At 3h post induction of sepsis, there was a significant fall in stroke volume and cardiac output. Septic animals mounted a significant tachycardia by 6h, which persisted at 24h. This paralleled the increase in core body temperature. By 48h, SV, CO, HR, and core temperature normalized among septic animals, and these remained stable untll 72h.
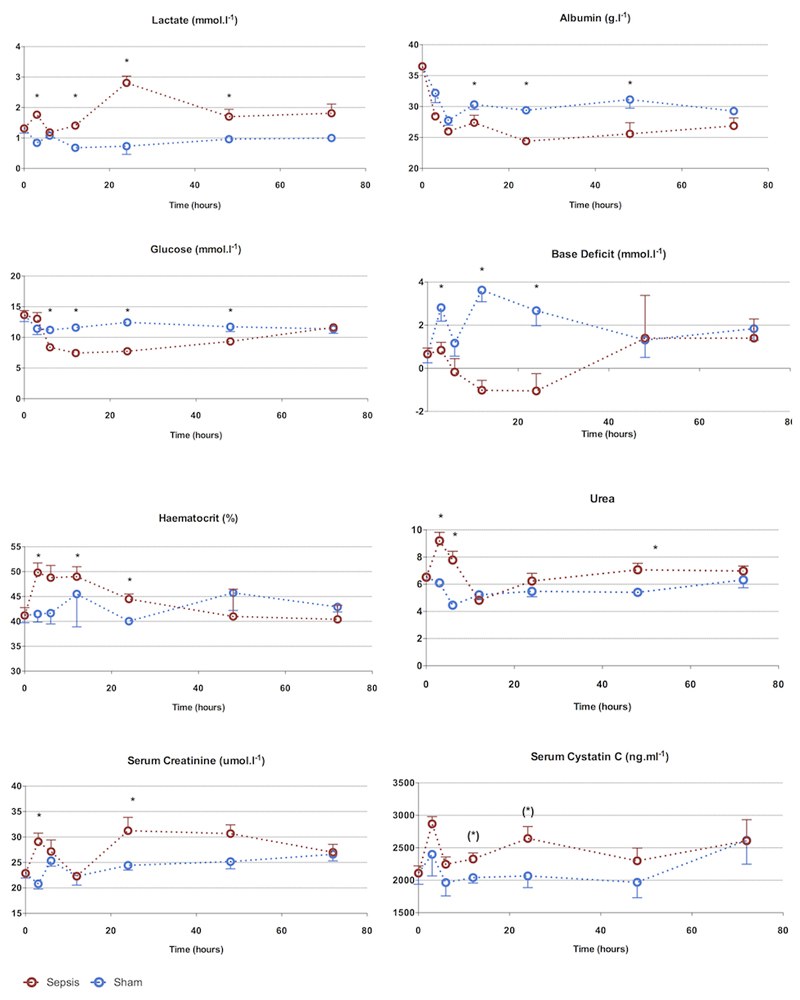
Biochemical markers (Figure 2)
Figure 2. 72-hour characterization of sepsis and recovery phases - biochemistry.
Biochemical changes occur from 3h. Apart from serum urea, changes are maximal at 24h followed by recovery to baseline values at 72h. The early rise in urea, creatinine, and lactate is corrected after fluid resuscitation, demonstrating early hemoconcentration.
Points and whiskers represent median and interquartile range respectively. (*: p<0.05 sham vs sepsis; (*): p=0.05-0.06 sham vs sepsis)
There was an early peak (3h) in serum urea and creatinine in septic animals. Alongside the significant rise in hematocrit at 6h, this suggests intravascular volume depletion, despite aggressive fluid loading. Once fluid resuscitation commenced (from 2h) there was a progressive fall in serum urea and creatinine that normalized by 6-12h. By 24h, serum urea and creatinine were significantly elevated. The peak serum creatinine level (30 μmol/l) was 1.5-fold above that of baseline. The increases in arterial lactate levels were phasic, with a rise at 3h, normalization at 6 h, a further peak at 24h and then a subsequent fall. The arterial base excess fell in the septic animals but normalized by 48h.The rise in serum cystatin C approached statistical significance by 12h and remained elevated at 24h. Compared to sham animals, serum albumin and glucose fell in the septic animals, reaching a nadir at 24h. This change was more pronounced in in the septic rats compared to sham-operated rats. Recovery occurred by 72h.
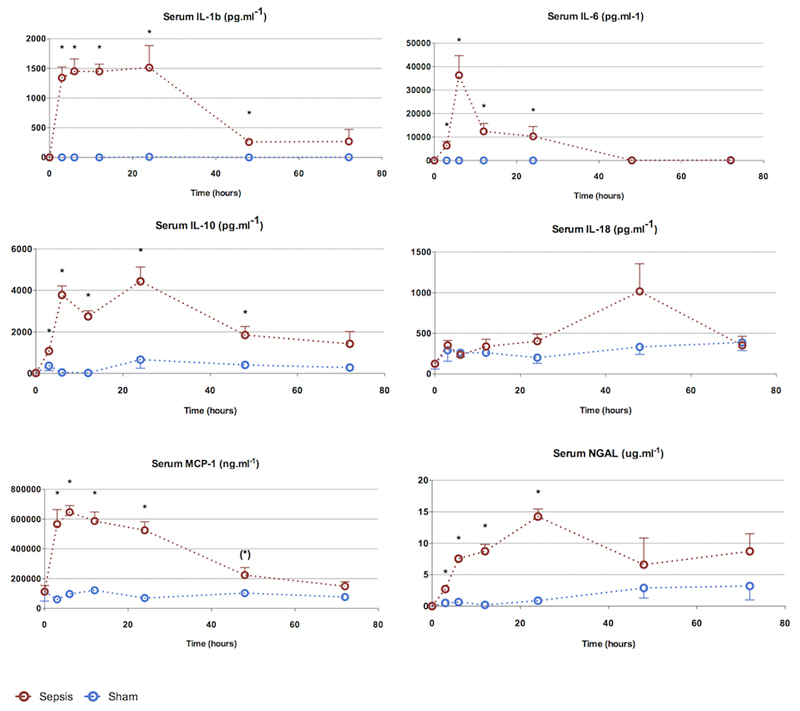
Serum cytokines (Figure 3)
Figure 3. Characterization of sepsis and recovery phases - serum cytokines.
The pro-inflammatory cytokine kinetic pattern is variable. IL-1β and IL-6 rise early (3h) and remain elevated until 24h. The anti-inflammatory cytokine IL-10 begins to rise by 3h but remains elevated through to resolution.
Points and whiskers represent median and interquartile range respectively. (*: p<0.05 sham vs sepsis; (*): p=0.05-0.06 sham vs sepsis)
Most pro-inflammatory cytokines were significantly elevated by 3h, including IL-1β, IL-6, MCP-1, and NGAL. Apart from IL-6, all these cytokines remained elevated for at least 24 h. The anti-inflammatory cytokine IL-10 was also significantly elevated at 3h. The earliest cytokine to peak was IL-1β at 3h; which remained elevated until 24h. Serum MCP-1 levels followed a similar pattern to IL-1β. IL-6, in contrast, peaked at 6h and fell sharply thereafter, to reach baseline levels by 48h. There was a trend towards an elevated IL-18 at 48h.
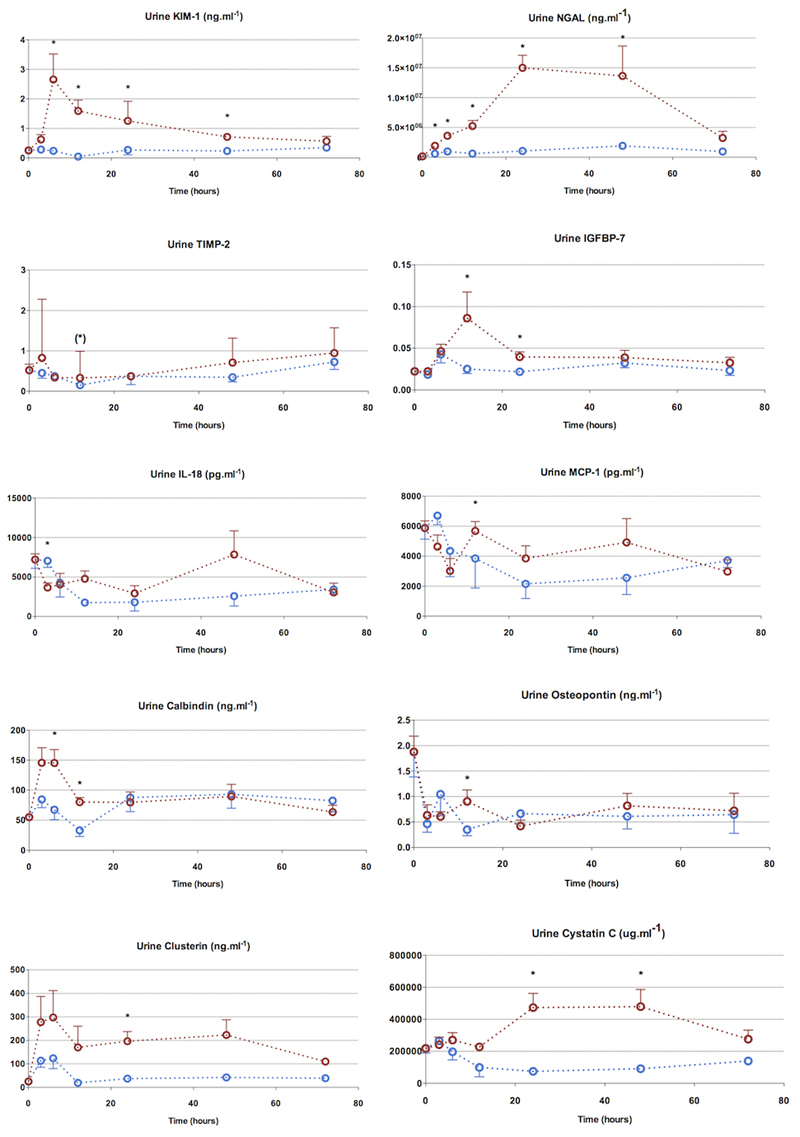
Urine biomarkers (Figure 4)
Figure 4. 72hour characterization - urine biomarkers.
Urine NGAL was the most sensitive marker, rising from 3h to 24h. Other biomarkers were also elevated before the rise in serum creatinine, though the magnitude of their rise was much less compared to NGAL. Urine IL-18 was not elevated in septic animals.
Points and whiskers represent median and interquartile range respectively. (*: p<0.05 sham vs sepsis; (*): p=0.05-0.06 sham vs sepsis)
Ten different urine biomarkers (KIM-1, NGAL, TIMP-2, IGFBP-7, IL-18, MCP-1, calbindin, clusterin, osteopontin, cystatin C) were measured in addition to serum biomarkers of renal function (serum urea, creatinine and cystatin C). All urine biomarkers related to tubular cell injury, apart from TIMP-2, were significantly altered to varied degrees and with different kinetics. All biomarkers returned to levels approaching those observed in sham animals with clinical recovery. Apart from urine osteopontin and IL-18, all other urine biomarkers were elevated and at an earlier time-point to serum creatinine (at 24h).
Urine NGAL was the earliest biomarker to rise (3h) with a sustained peak lasting from 24-48h. Urine KIM-1 and calbindin peaked at 6h, and fell thereafter, with calbdinin reaching baseline values by 24h and KIM-1 by 72h. Although urine clusterin rose early (3h), it lacked discriminatory values due to variability in values. Urine cystatin C, a marker of GFR and intact tubular reabsorption, was raised between 24-48h in septic animals.
Urine IGFBP-7, a marker of cell cycle arrest, was significantly elevated at 12h. This followed the rise seen in tubular injury markers but preceded the rise in functional markers of filtration (i.e. serum creatinine and cystatin C). TIMP-2, another cell cycle arrest marker, was also elevated at 12h, but only approached statistical significance. The fall in urinary levels of KIM-1 and calbindin at 24h appear to predict renal recovery. Although this time-point coincided with a rise in serum creatinine, there was associated improvement in hemodynamics and a fall in pro-inflammatory cytokines (IL-1β, IL-6, IL-18).
In summary, some urine biomarkers including KIM-1 and calbindin rose early and then showed an early fall. On the other hand, NGAL also rose early, but peaked later, and remained elevated till clinical recovery. Cell cycle arrest markers rose after the injury markers and fell prior to clinical recovery. Urine cystatin C rose later, at the same time as serum creatinine (24h), suggestive of decreased renal functionality. Urine MCP-1, osteopontin, and IL-18 were elevated at various points in the clinical course but did not demonstrate any clear pattern of rise and fall.
As with current clinical use, it is unclear to what extent the urine biomarkers reflect what is filtered from the circulation into the urine, as opposed to de novo production within the kidney. There was no correlation between paired urine and serum levels of IL-18, MCP-1, or cystatin C. A modest positive correlation was seen between urine and serum NGAL values (r2 = 0.713, p<0.001), even when including septic animals only (r2 = 0.654, p<0.001).
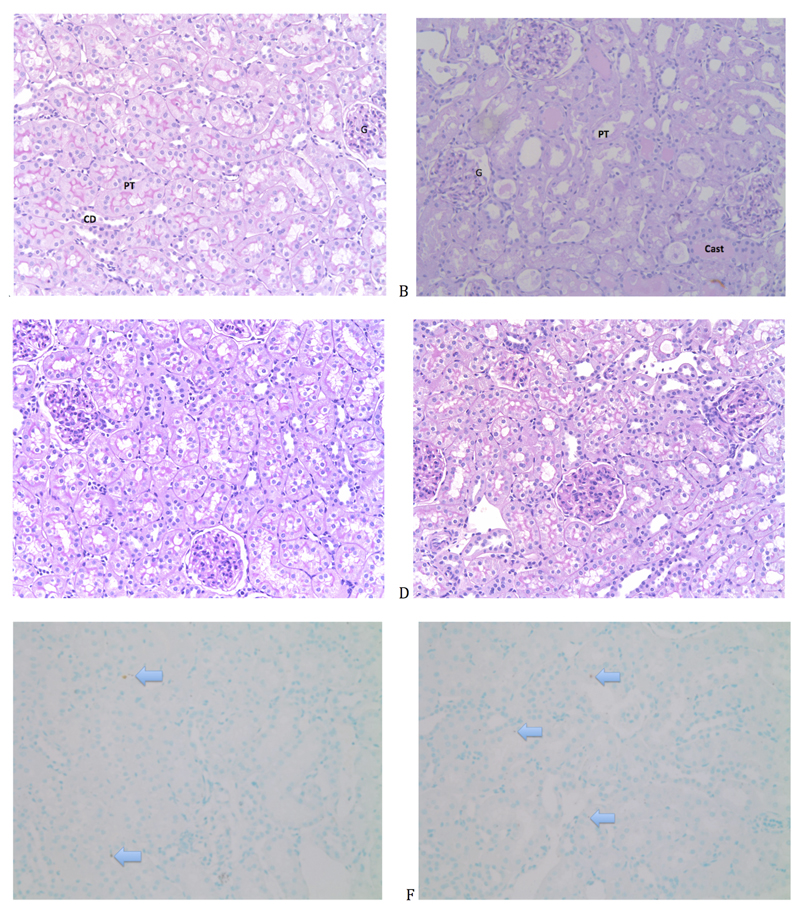
Renal histology (Figures 5)
Figure 5. Histological assessment of rat kidneys (Magnification x 20).
A. Naive renal tissue without any significant damage. B. Renal tissue obtained from a haemorrhage-reperfusion model (A. Dyson, UCL), showing several characteristics of acute tubular injury including dilated tubules with loss of brush border, ischemic glomeruli and tubular casts. C. Kidney section from a 24h sham-operated rat. D. Kidney section from a 24h septic rat. E. TUNEL stain of naïve renal tissue shows 2 apoptotic cells per x20 field. Apoptotic cells stain dark brown (arrow). F. TUNEL stain of 24hr septic renal tissue shows occasional apoptotic cells. Apoptotic bodies seen mainly in proximal tubular epithelial cells. (x20 magnification). (PT: Proximal tubule. G: Glomerulus. Cast: Tubular cast. Arrow: TUNEL positive cell)
The degree of injury at 24h was relatively mild. Predominant findings included mild tubular dilatation and brush border loss. Tubular injury was patchy, among areas of normal histology. By comparison, histology is shown of a haemorrhage-reperfusion model performed in the lab in similarly aged male Wistar rats (Fig 5B;). TUNEL staining revealed minimal presence of cell death, with an average of two TUNEL positive cells per 20x magnification field. Where present, TUNEL positive cells were within proximal tubular epithelial cells (Figure 5).
Discussion
Our study aim was to measure temporal changes in a panel of biomarkers measured from the onset of sepsis through to recovery in a long-term fluid-resuscitated model of fecal peritonitis. This model demonstrates many hemodynamic, biochemical, and immunological features consistent with clinical sepsis. As with human septic AKI, renal histology demonstrated minimal structural injury or cell death; influx of inflammatory cells was not seen. We report novel data showing an early change in markers of renal injury (urine NGAL, KIM-1, calbindin), followed by a marker of cell cycle arrest (urine IGFBP7) and, finally, by functional markers of filtration (serum creatinine and cystatin C) (Supplemental Digital Content – Figure 1). Urine NGAL was the most sensitive marker among those studied, rising from 3 to 24h. The rise of the functional biomarker, serum cystatin C, at 12h implies a fall in GFR, while the concurrent fall in serum creatinine is suggestive of decreased creatinine production.
Serum urea and creatinine were initially elevated at 3h and 6h, and fell towards baseline values at 12h with IV fluid resuscitation. This is consistent with early hemoconcentration followed by a dilution effect. A correction factor can be applied to measured serum creatinine to correct for fluid administration (7) as acute hemodilution may mask the creatinine rise in early AKI (8). However, applying a correction factor for cumulative fluid balance over periods longer than a few hours lacks both a physiological rationale and objective evidence of accuracy (9). After a large fluid bolus that increased circulating volume by 25%, 24h serum creatinine was only 2% below expected (9). The rise in serum cystatin C (consistent with a decrease in GFR) from 6h to 12h with concurrent falls in serum creatinine may be a consequence of decreased creatinine production (10). As with serum creatinine, sepsis decreases serum cystatin C production and increases non-renal clearance (11). However, serum cystatin C had a faster rise and peaked more rapidly than creatinine. As such, serum cystatin C detects AKI early and better reflects inulin GFR in CLP-induced murine sepsis.
The temporal changes in cytokine levels in our model was similar to a cohort study of 1886 subjects hospitalized with community-acquired pneumonia (12). There was an early peak of both pro- and anti-inflammatory cytokines followed by a decline in the pro-inflammatory cytokine profiles and persistence of the anti-inflammatory cytokine IL-10 though to recovery.
FDA- approved AKI biomarkers urine TIMP-2 and IGFBP-7 may be superior to NGAL in diagnosing AKI early in critically ill patients (13, 14). TIMP-2 and IGFBP-7 measured early in the setting of critical illness may also identify patients with AKI at increased risk of mortality or receipt of RRT in the subsequent nine months (15). In a caecal ligation and puncture (CLP) model of sepsis, the combination of TIMP-2 and IGFBP-7 has greater sensitivity in diagnosis of AKI compared to serum creatinine (6). However, the kinetics of these biomarkers in renal recovery has not been described.
Urine IL-18 is less promising than urine NGAL in septic AKI (13) and we report here similar findings. Two markers of distal tubular injury, calbindin and osteopontin, have been previously evaluated in septic AKI. Urine calbindin was elevated at 6-12 h whereas urine ostopontin did not differ between septic and sham animals. Urine MCP-1 has also not been characterized in septic AKI; levels were significantly elevated between 12-18h.
A transient and modest, albeit statistically significant rise, was seen in urine osteopontin levels at 12h. Within the normal kidney, osteopontin is mainly present in the loop of Henlé and distal nephron (16) but, in our model, proximal tubular injury predominated. In a renal ischemia-reperfusion injury model, the distribution of osteopontin in proximal (PTECs) and distal (DTECs) tubular epithelial cells differed (17). While DTECs showed an early and persistent increase in osteopontin, the rise seen in PTECs was delayed and mostly associated with morphological regeneration. This suggests osteopontin may promote recovery via modulation of infiltrating cells and local responses by the PTEC. Ours, we believe, is the first study to measure urine osteopontin in septic AKI.
Consistent with other studies in both patients and animal models (18–20), the renal histology in our model shows disproportionately minimal parenchymal injury and apoptosis for the degree of functional impairment measured. There was no evidence of interstitial hypercellularity at any point to suggest significant immune cell infiltration.
Our pre-clinical model utilized a homogenous population of relatively young rats receiving an identical insult 24 hours after anesthesia and instrumentation. Patients with sepsis have a more variable genetic make-up, are usually older, with co-existing comorbid illnesses, and are receiving other nephrotoxic and renal physiology-modifying interventions. Other than hemodynamics and temperature, serial measurements were not made in the same animal as each time-point represents the terminal experiment for collection of blood, renal tissue and urine samples. We therefore cannot plot the trajectory of variables such as urine output or recovery from anuria over time. As the most unwell animals were anuric, urine biomarkers could not be measured in these animals.
Despite ample fluid resuscitation and becoming febrile, the rats in our peritonitis model do not develop a hyperdynamic circulation. We have previously shown in this model significant myocardial depression yet maintained cardiac output (21). Many large animal models use an infusion of endotoxin or intravenous administration of live bacteria (rather than the more clinically representative fecal peritonitis insult), and these may produce a different circulatory/inflammatory profile
We avoided antibiotic use in our study to avoid potential confounding from direct nephrotoxicity, and renal impairment secondary to increased inflammation secondary to antibiotic use (22). We thus did not wish to confound the biomarker data in our study with a potential additional impact of antibiotics, which, may add nephrotoxicity. Despite the non-use of antibiotics, 80% of rats recovered with fluid resuscitation only. Similar data have been reported by Hollenberg et al in a mouse CLP model (23). Of note, the outcome effectiveness of antibiotics is age-dependent (24).
Serum creatinine in the septic animals rose by only 50%, less than that seen clinically. However, in this fluid-resuscitated model, animals with a >50% rise in serum creatinine at 24h tended to not survive much longer. In pilot studies the creatinine rise seen at 24h could be reached by 6h in the absence of fluid resuscitation (data not shown). However, in these non-resuscitated animals, mortality rates were both very high and occurred much earlier, preventing study of the recovery phase.
Normalization to urinary creatinine concentration improved the prediction of developing AKI and outcome among critically ill patients, but provided no advantage in diagnosing established AKI (25). Similar findings were described in a rat model of drug-induced AKI (26). In this study, biomarker levels could not be corrected to urine creatinine concentration in many animals due to limited or absent urine output, particularly in the most severely affected.
We did not compare these biomarkers against other inflammatory, non-septic insults, nor did we try to differentiate systemic from local production of the biomarker. Because of the need to terminally anaesthetize the animals to obtain blood, urine and tissue samples, we did not monitor biomarker levels in the same animal over time and thus could not assess the ability to prognosticate for the development of AKI.
No specific intervention currently exists for septic AKI. Intuitively, initiating therapies early would be of greater benefit, but this remains speculative. ‘Time zero’, the time of onset of AKI, is rarely known in patients. If the biomarker pattern we observed in our rat model holds true for patients this may aid selection for interventions and potentially enhance the likelihood of therapeutic benefit. The temporal relationship of biomarker change described may shed some light into pathophysiological mechanisms.
Supplementary Material
There is a distinct temporal change markers of renal injury (KIM-1), followed by a marker of cell cycle arrest (urine IGFBP7) and, finally, by functional markers of filtration (serum creatinine and cystatin C).
Acknowledgments
Funding: Dr. Arulkumaran and his Institution (UCL) received grant support from the Wellcome Trust Clinical Research Training Fellowship (grant in support of this project in part, including Dr. Arulkumaran’s salary). Additional funding for the biomarker analysis was provided by an UK Intensive Care Society New Investigator Award awarded to Dr. Arulkumaran
Copyright form disclosure: Dr. Arulkumaran received support for article research from Wellcome Trust/COAF; he and his Institution (UCL) received grant support from the Wellcome Trust Clinical Research Training Fellowship (grant in support of this project in part, including Dr. Arulkumaran’s salary); and additional funding for the biomarker analysis was provided by an UK Intensive Care Society New Investigator Award awarded to Dr. Arulkumaran. Dr. Kellum’s institution received funding from Astute Medical (consulting and grant support), and he has licensed unrelated technologies through the University of Pittsburgh to Astute Medical. Dr. Unwin received support for article research from Wellcome Trust/COAF. Dr. Tam’s institution received funding from AstraZeneca (research project grant); he received support for article research from Wellcome Trust/COAF, and disclosed receiving a Case Fellowship joint award from BBSRC and GSK on P2x7 receptor in eye disease; he disclosed he is the chief investigator of the randomized controlled trial of Syk inhibitor in IgA nephropathy, has been on the Advisory Board of MedImmune, has a consultancy agreement with Rigel Pharmaceuticals, and has also received research grants from GSK; he disclosed he is the co-supervisor of a Welcome Trust Clinical Research Training Fellow for Dr Nishkantha Arunkumaran, who is the first author of this paper. Dr. Singer’s institution received funding from Wellcome Trust (PhD studentship), UK Intensive Care Society Young Investigator Award, from various academic grants (e.g Wellcome Trust, MRC, European Union), and from different companies that are paid to the institution to support research activity (e.g. with Abbott, Deltex, DSTL, Magnus Oxygen, NewB Innovation, Oxford Optronix, Probe Scientific) he has performed consultancy/advisory board work or received speaker fees from Bayer, Biotest, Deltex, Fresenius, Merck, Pfizer, SOBI, and he is Scientific Officer for Magnus Oxygen; he received support for article research from Wellcome Trust/COAF. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Reprints:
For information regarding this article/ reprints, m.singer@ucl.ac.uk
Conflict of interest:
JAK has received consulting fees and grant support from Astute Medical and has licensed unrelated technologies through the University of Pittsburgh to Astute Medical.
References
- 1.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 3.Srisawat N, Murugan R, Lee M, et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80:545–552. doi: 10.1038/ki.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudiger A, Dyson A, Felsmann K, et al. Early functional and transcriptomic changes in the myocardium predict outcome in a long-term rat model of sepsis. Clin Sci. 2013;124:391–401. doi: 10.1042/CS20120334. [DOI] [PubMed] [Google Scholar]
- 5.Dyson A, Rudiger A, Singer M. Temporal changes in tissue cardiorespiratory function during faecal peritonitis. Intensive Care Med. 2011;37:1192–1200. doi: 10.1007/s00134-011-2227-z. [DOI] [PubMed] [Google Scholar]
- 6.Peng ZY, Zhou F, Kellum JA. Cross-species validation of cell cycle arrest markers for acute kidney injury in the rat during sepsis. Intensive Care Med Exp. 2016;4:12. doi: 10.1186/s40635-016-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu KD, Thompson BT, Ancukiewicz M, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665–2671. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prowle JR, Leitch A, Kirwan CJ, et al. Positive fluid balance and AKI diagnosis: assessing the extent and duration of “creatinine dilution”. Intensive Care Med. 2015;41:160–161. doi: 10.1007/s00134-014-3538-7. [DOI] [PubMed] [Google Scholar]
- 10.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–1221A. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leelahavanichkul A, Souza AC, Street JM, et al. Comparison of serum creatinine and serum cystatin C as biomarkers to detect sepsis- induced acute kidney injury and to predict mortality in CD-1 mice. Am J Physiol Ren Physiol. 2014;307:F939–F948. doi: 10.1152/ajprenal.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honore PM, Nguyen HB, Gong M, et al. Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Risk Stratification of Acute Kidney Injury in Patients With Sepsis. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyner JL, Shaw AD, Chawla LS, et al. Tissue Inhibitor Metalloproteinase-2 (TIMP-2)IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol. 2015;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Sakatsume M, Nishi S, et al. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001;60:1645–1657. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 17.Persy VP, Verstrepen WA, Ysebaert DK, et al. Differences in osteopontin up-regulation between proximal and distal tubules after renal ischemia/reperfusion. Kidney Int. 1999;56:601–611. doi: 10.1046/j.1523-1755.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 18.Langenberg C, Bagshaw SM, May CN, et al. The histopathology of septic acute kidney injury: a systematic review. Crit Care. 2008;12:R38. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerolle N, Nochy D, Guerot E, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 20.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan Y-L, Orie NN, Dyson A, et al. Inhibition of vascular adenosine triphosphate-sensitive potassium channels by sympathetic tone during sepsis. Crit Care Med. 2012;40:1261–8. doi: 10.1097/CCM.0b013e31823da98d. [DOI] [PubMed] [Google Scholar]
- 22.Peng Z-Y, Wang H-Z, Srisawat N, et al. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit Care Med. 2012;40:538–43. doi: 10.1097/CCM.0b013e31822f0d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollenberg SM, Dumasius A, Easington C, et al. Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med. 2001;164:891–895. doi: 10.1164/ajrccm.164.5.2010073. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull IR, Wizorek JJ, Osborne D, et al. Effects of Age on Mortality and Antibiotic Efficacy in Cecal Ligation and Puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Ralib AM, Pickering JW, Shaw GM, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol. 2012;23:322–333. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonomura Y, Uehara T, Yamamoto E, et al. Decrease in urinary creatinine in acute kidney injury influences diagnostic value of urinary biomarker-to-creatinine ratio in rats. Toxicology. 2011;290:241–248. doi: 10.1016/j.tox.2011.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
There is a distinct temporal change markers of renal injury (KIM-1), followed by a marker of cell cycle arrest (urine IGFBP7) and, finally, by functional markers of filtration (serum creatinine and cystatin C).