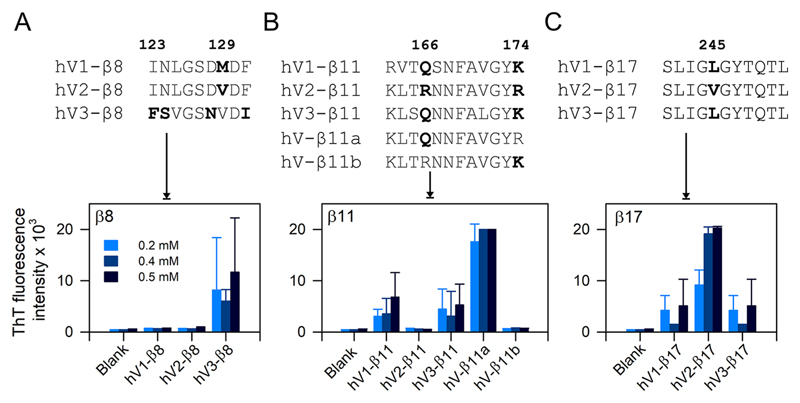
Figure 3.
Reverse-mapping strategy identifies sequence-driven aggregation propensity of hVDAC peptides. (top) Multiple sequence alignment of β8, β11, and β17 showing differences in the primary peptide sequence. Amino acid numbering is based on hV1/hV3. (bottom) ThT fluorescence histograms are highlighting the differences in aggregation tendency in pH 7.2. (A) hV3-β8 shows increased aggregation tendency, whereas aggregation of hV1- and hV2-β8 is negligible. (B) Comparison of aggregation tendency of β11 permutants shows considerable differences for each isoform. Hybrid peptides of β11 (β11a and β11b) prove that a single residue substitution in hV2-β11 (R166 → Q in hV-β11a) renders this peptide highly sensitive to aggregation. (C) A conserved single amino acid difference between hV1/hV3-β17 and hV2-β17 (245L → V) determines the aggregation tendency. hV2-β17 show a 2-fold increase in ThT intensity compared to hV1/hV3-β17. All three examples highlight the efficiency of reverse mapping to demarcate peptide aggregation propensity. Error bars represent SD from at least three independent experiments.