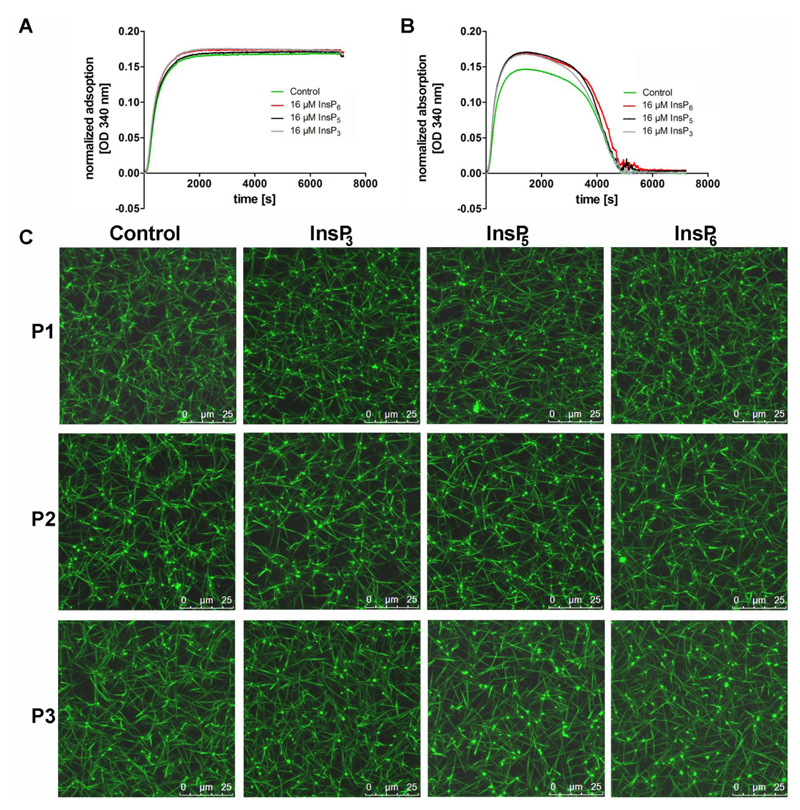
Fig. 5. Fibrin polymerization and fibrinolysis in the presence of InsPs.
(A) For turbidity analysis of fibrin polymerization, fibrinogen purified from human plasma was incubated with 16 μM InsP3 (grey line), InsP5 (black line) or InsP6 (red line) in the presence of 5 mM CaCl2 and 0.1 U/ml human α-thrombin. The control without InsPs is shown by the green line. (B) Fibrinolysis was measured employing an adapted turbidity assay by addition of t-PA (100 pM) and plasminogen (0.24 μM) to the reaction described in (A). Changes in absorbency were monitored at 340 nm, every 12 s for 2 h at room temperature, using a microtiter plate reader. (C) Human plasma from three different donors (P1-P3), CaCl2 (5 mM), Alexa Fluor 488 labeled fibrinogen (10%) and 16 μM InsP3 (D), InsP5 (E) or InsP6 (F) were diluted in TBS. After addition of human α-thrombin (0.175 U/ml final concentration), the reaction mixture was immediately transferred into the channel of an Ibidi μ-slide VI0.4. After fibrin network formation was completed, Z-stacks with 20 slices of 1 μm were recorded at room temperature using a confocal microscope (TCS SP5, Leica, Wetzlar, Germany) equipped with an HC PL APO CS2 63.0x1.40 OIL UV objective (Leica) and the following settings: zoom 3x, image size of 512 × 512, laser power of the 488 lasers was set 5 %. 3D reconstruction was performed using the ImageJ software [27]. The scale bars represent 25 μm.