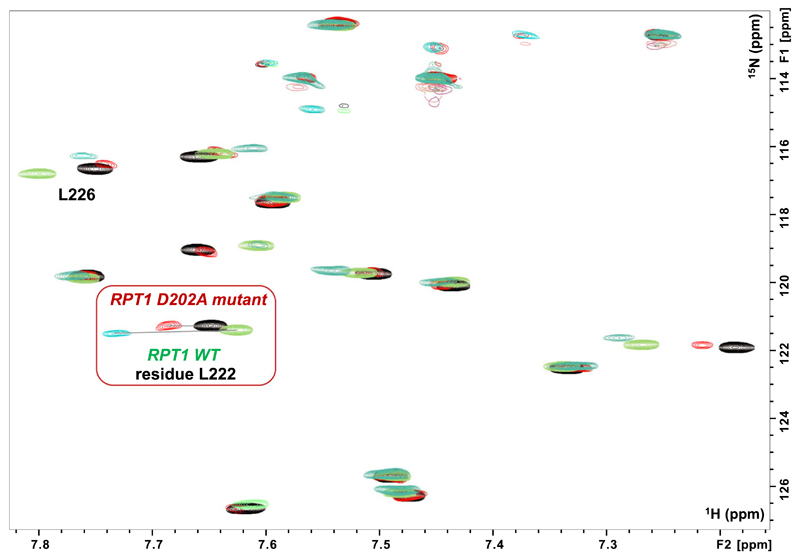
Fig. 6.
Binding of the MYC:MAX dimer to the INI1 D202A-mutant. Overlay of a region of the 1H,15N HSQC spectra of RPT1 INI1 WT without (blue gray) and with (green) unlabeled MYC:MAX bHLHZip dimer (ratio 1 : 1), overlaid with the overlay of the same region of the 1H,15N HSQC spectra of RPT1 INI1 D202A-mutant without (black) and with (red) unlabeled MYC:MAX bHLHZip dimer (ratio 1 : 1). Highlighted in the red square is the residue L222 which undergoes the largest change in chemical shift upon binding of MYC:MAX complex to both INI1 RPT1 WT and the D202A mutant. Comparison of the same ratio (1 : 1) illustrates the lower affinity of the DA mutant as the change in chemical shift of L222 is significantly less.