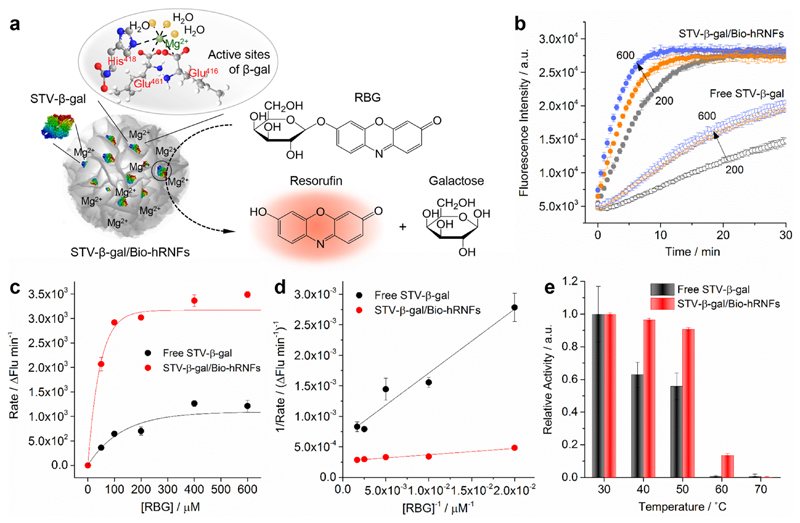
Figure 6.
(a) Schematic illustration of the STV-β-gal activity assay using the RBG substrate. The catalytic activity of STV-β-gal was measured by monitoring the red fluorescence signal of resorufin with an emission maximum at 584 nm. The active sites of β-gal containing a Mg2+ ion bound to three amino acid residues (His418, Glu416, and Glu461) and three water molecules are shown. (b) Catalytic kinetics of free STV-β-gal and STV-β-gal/Bio-hRNFs at different enzyme concentrations (200, 400, and 600 ng/mL). (c) Michaelis–Menten and (d) Lineweaver–Burk plots of free STV-β-gal and STV-β-gal/Bio-hRNFs. (e) Relative activity of free STV-β-gal and STV-β-gal/Bio-hRNFs at various temperatures (30–70 °C). The relative enzymatic activity was obtained by normalizing the fluorescence signals of both free STV-β-gal and STV-β-gal/Bio-hRNFs to that of free STV-β-gal or STV-β-gal/Bio-hRNFs heated at 30 °C, respectively. Results represent mean ± s.d. of three independent experiments.