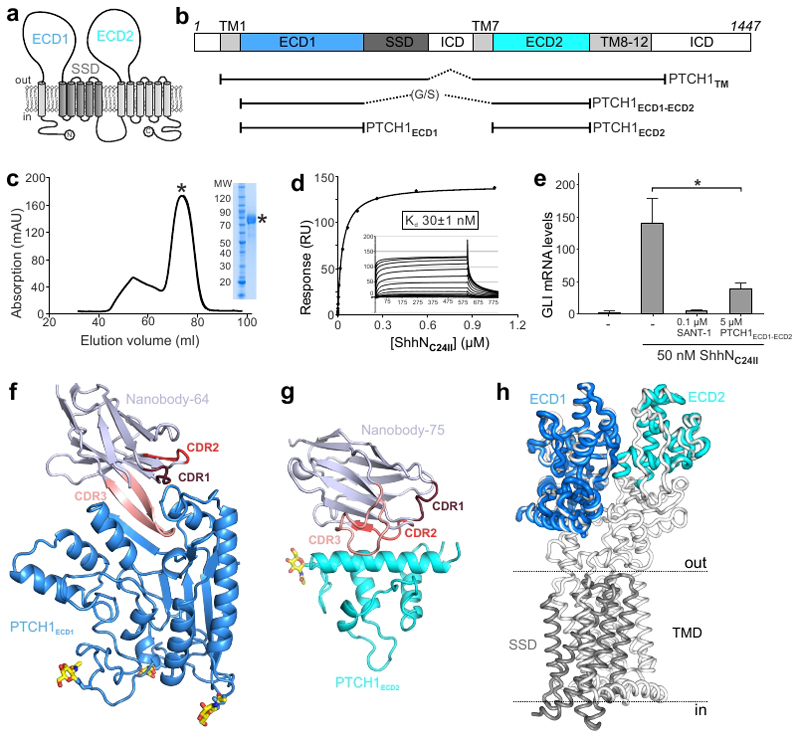
Figure 1. Structural and function characterization of PTCH1-nanobody interactions.
a, The pseudo-symmetric domain architecture of PTCH1: two 6-TM segments with extracellular domains (ECD1 and ECD2) interposed between the first two TM helices of each segment. The SSD, composed of TM helices 2-6, is marked in gray. b, Composition of the various protein constructs used in this study. c-e, Characterization of PTCH1ECD1-ECD2 used as an antigen to immunize Llamas. c, Typical SEC purification and corresponding SDS-PAGE of pooled fractions of PTCH1ECD1-ECD2. d, SPR equilibrium binding experiment between PTCH1ECD1-ECD2 (ligand) and non-lipidated ShhNC24II (analyte). This experiment was independently repeated 3 times with similar results. e, SHH signalling assay in mouse NIH-3T3 cells. Normalized Gli1 mRNA expression was used to assess SHH signalling activity by RT-qPCR after stimulation with purified ShhNC24II. Error bars denote SEM of 3 independent experiments. Statistical value is p=0.0387 determined by ordinary one-way ANOVA with Turkey’s multiple comparisons test. Sant-1 is a HH signalling inhibitor acting downstream of PTCH1. PTCH1ECD1-ECD2 acts as a ligand trap to inhibit Hh signalling. f-g, Cartoon representations of the high-resolution crystal structures of the PTCH1ECD1-NB64 (f) and PTCH1ECD2-NB75 (g) complexes. The complementary determining regions (CDRs) of the nanobodies are highlighted and N-linked glycans are shown in atomic colouring (carbon: yellow, oxygen: red, nitrogen: blue). h, Superposition of the PTCH1ECD1 and PTCH1ECD2 crystal structures on the previously determined cryo-EM PTCH1TM structure (PDB 6E1H16).