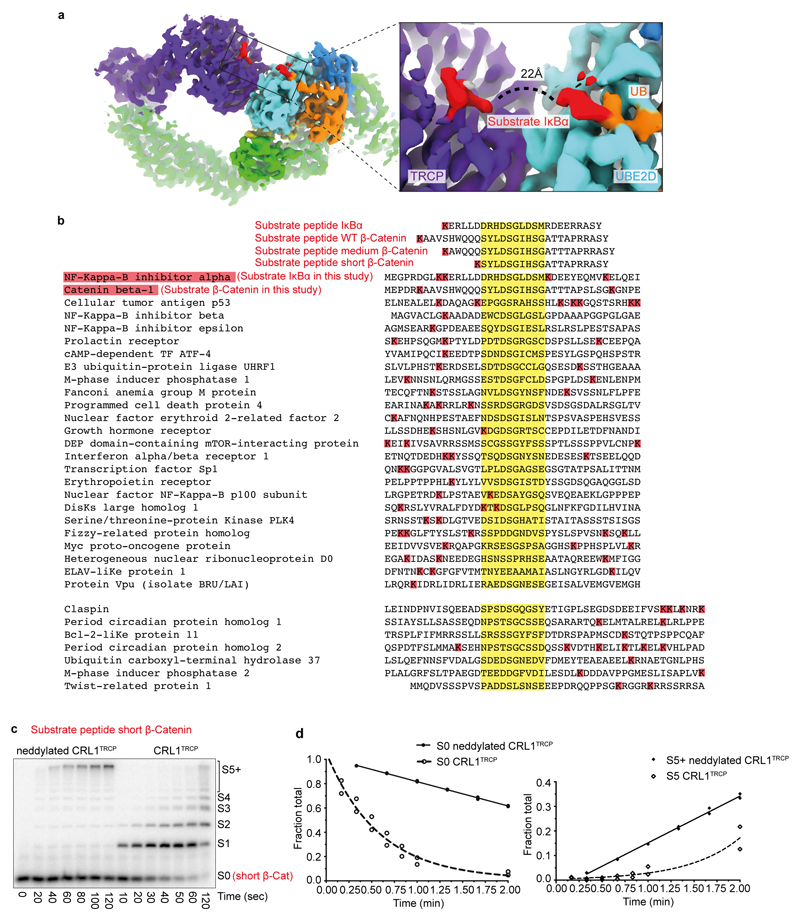
Extended Data Figure 6. Geometry between phosphodegron and acceptor in structure, substrates, and ubiquitylation.
a, Cryo EM density highlighting the relative placement of substrate degron and UBE2D~UB active site. The ~22Å distance between UBE2D~UB active site and the phosphodegron of β-TRCP-bound substrate requires at least 6 intervening residues in a substrate. b, Alignments for several reported β-TRCP substrates32, highlighting the degron sequence (yellow) and nearby lysines (red). Also shown are sequences of peptide substrates with a single acceptor Lys that were used in kinetics analyses. The peptide sequences were derived from IκBα, and from β-catenin with varying spacers between phosphodegron and acceptor Lys: WT β-catenin peptide, “medium” β-catenin peptide with lysine corresponding to IκBα, and “short” β-catenin peptide with a lysine 5 residues upstream of the N-terminal phosphoSer in the degron, which would be too short to bridge the structurally-observed distance between the phosphodegron binding site on β-TRCP and UBE2D catalytic Cys in the ubiquitylation active site. c, Representative autoradiogram (n = 2) of SDS-PAGE gel showing products from indicated time points of ubiquitylation reactions under multi-turnover conditions with either neddylated or unneddylated CRL1β-TRCP and radiolabeled short β-catenin peptide substrate. The amount of short β-catenin peptide modified by neddylated CRL1β-TRCP and UBE2D is too low in the single-encounter ubiquitylation reaction to allow quantification of kinetic parameters, yet, product formation is apparent under multi-turnover conditions and shows that most products are heavily ubiquitylated. d, Plots fitting consumption of unmodified short β-catenin peptide substrate (S0) compared to formation of polyubiquitin chains with 5 or more UBs (S5+) from reactions as in panel c. The symbols show the data from independent experiments (n = 2 technical replicates).