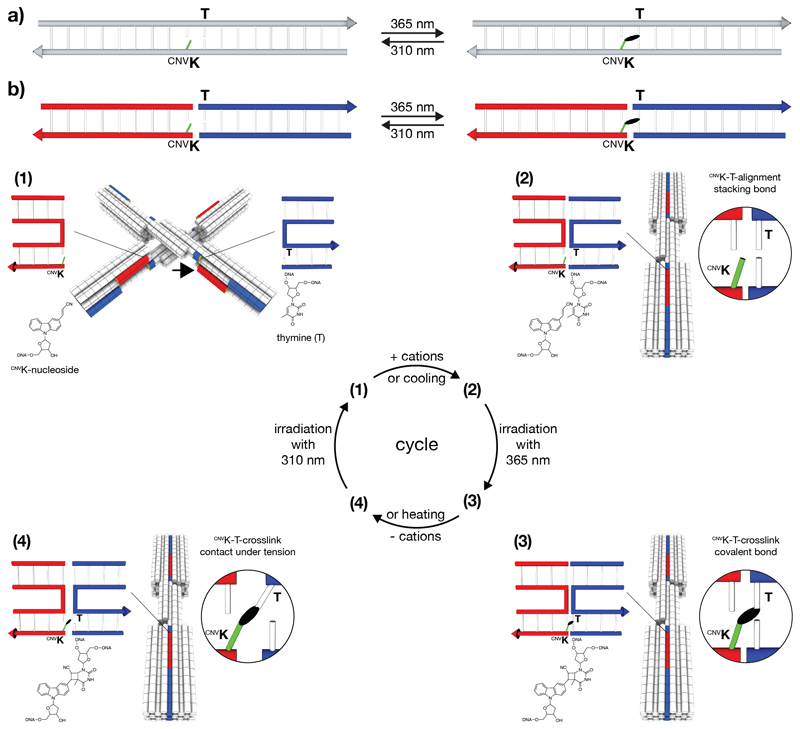
Figure 1.
Schematics of photo-crosslinking and photo-splitting of cnvK-modified nucleosides across stacking contacts. a) Covalent photo-crosslinking within the context of a continuous DNA double helix. The cyanovinyl group of the cnvK-modified nucleobase (highlighted in light green) can be covalently linked to the thymine on the ‘-1’ position (from 5’ to 3’) of the complementary strand. The black ellipsoid represents the covalent bond. b) Covalent photo-crosslinking within the context of a stacking contact in DNA assemblies. The stacking contact consists of double helical protrusions (highlighted in red) and double helical recessions (highlighted in blue) that form discontinuous DNA double helices in the bound state. (1) – (4): Scheme of photo-crosslinking and photo-splitting using the DNA origami switch object. (1) Open conformation of the switch object under low ionic strength conditions. The cnvK moiety and the thymine (T) are separated in space. The black arrow indicates the position of the second cnvK-modification (Figure S1). (2) Closed conformation of the switch object under high ionic strength conditions. The cnvK moiety and the thymine are co-localized in space. (3) Formation of the covalent bond between the cnvK and T upon 365 nm irradiation. (4) The stacking contact is under tension when decreasing the cation concentration. Splitting the cnvK-T-covalent bond can be triggered upon 310 nm irradiation.