Abstract
Background
Sepsis is an important cause of mortality globally, though population incidence estimates from low income settings, including sub-Saharan Africa (sSA), are absent. We aimed to estimate sepsis incidence burden using routinely available data from a large urban hospital in Malawi.
Method
We linked routine-care databases at Queen Elizabeth Central Hospital, Blantyre, Malawi, to provide admission and discharge data for 217,149 adults from 2013-2016. Using a definition of sepsis based on systemic inflammatory response syndrome (SIRS) criteria, and Blantyre census population data, we calculated population incidence estimates of sepsis and severe sepsis and used negative binomial regression to assess for trends over time. Missing data were multiply imputed with chained equations.
Results
We estimate that the incidence rate of emergency department-attending sepsis and severe sepsis in adults was 1772 per 100,000 person-years (95% CI 1754-1789) and 303 per 100,000 person-years (95% CI 295-310) respectively, between 2013 and 2016, with a year-on-year decrease in incidence. In-hospital mortality for patients admitted to the hospital with sepsis and severe sepsis was 23.7% (95% CI 22.7-24.7%) and 28.1% (95% CI 26.1 – 30.0%) respectively, with no clear change over time.
Conclusions
Sepsis incidence is higher in Blantyre, Malawi, than in high-income settings, from where the majority of sepsis incidence data derive. Worldwide sepsis burden is likely to be underestimated, and data from low income countries are needed to inform the public health response.
Keywords: sepsis, epidemiology, Africa south of the Sahara, low resource setting
Introduction
Sepsis, recently redefined as a syndrome of life-threatening organ dysfunction triggered by infection[1], is a significant public health problem, with an estimated 31.5 million cases worldwide, and 5.3 million deaths[2]. However, these figures are at best rough approximations: population incidence data for low-income settings, including sub-Saharan Africa (sSA), are absent, and there are reasons to suspect that the burden of sepsis may be higher in low-income settings. In Africa, febrile illness is common: malaria, for example, had an estimated incidence of 219.4 cases per 1000 person-years at risk in 2017[3]. Though sepsis is not included in global burden of disease estimates, infections such as pneumonia[4], meningitis[5] and typhoid fever[6] have persistently higher incidence rates in low and middle income countries than in high-income countries. In addition, the few available cohort studies suggest that that the case fatality rates of sepsis in sSA are higher than in high income settings[7,8].
By using a routinely-collected dataset of over 200,000 emergency department attendances from a single urban African centre, we aimed to estimate the overall and age-stratified population incidence of sepsis in Blantyre, to describe changes over a four-year period, and to estimate inpatient sepsis case fatality rate.
Methods
Setting and data sources
Malawi is a low-income country in South-East Africa. It has a high burden of HIV and TB as defined by the WHO with an adult (age 15-49) HIV prevalence of 9.6% in 2016[9]. Healthcare is free to all at the point of delivery (including tuberculosis and malaria treatment) and free antiretroviral (ART) treatment has been available since 2004. Malawi has a hot, wet season from November to March, and well recognized seasonal diseases patterns. Malaria is endemic but with peak incidence during the rainy season from November to May[10], with peaks of invasive Salmonella infection during and after the rainy season[11], and invasive pneumococcal disease in the dry season[12].
Queen Elizabeth Central Hospital, Blantyre, Malawi, has 1000 beds, and is the only government hospital providing free inpatient medical care to the city of Blantyre (population 800,024 at the 2018 census). The majority of adult inpatients are HIV-infected; previous studies have found 75-90% of patients with sepsis, fever, meningitis or pneumonia to be HIV-infected[13–16]. Adults are admitted via a dedicated emergency department (ED). Despite its tertiary facility status, most attendees are either primary presentation or referrals from primary health care clinics; inter-hospital transfers are not common. In 2011, triage was introduced, and an electronic patient registration system was established in September 2012, recording basic patient demographics and vital signs before directing staff to the most appropriate triage category. Additionally, an independent limited electronic patient record has been used to record vital status and diagnostic information at patient discharge for those admitted to the wards (but not those directly discharged from ED)[17].
We used anonymized data from both electronic systems, and matched patients on unique system identifiers and dates of attendance for a four year period (2013-2016). HIV status and details of any investigations (blood tests, radiology or microbiology) were not available. We included all ED-attending patients aged 14 years or over, and all patients in electronic discharge record which could be linked to an admission record.
For Malawi population data we retrieved the Malawian census data for Blantyre city for 2008 and 2018 from the National Statistical Office of Malawi[18]. To extrapolate between these two time points we fitted a log transformed time curve using a linear model. Age distribution within Blantyre city was not available for 2018: we extrapolated age-specific denominators from national data on pooled urban populations.
Statistical analysis
We defined sepsis using a modified Sepsis-2 definition[19] as fever (>38°C) or hypothermia (<36°C) plus one of tachycardia (heart rate >90/min) or tachypnoea (respiratory rate >22/min). We defined severe sepsis as sepsis plus one of shock (systolic blood pressure <90mmHg), hypoxia (SpO2 <90%) or reduced conscious level (below A on the AVPU scale). Due to the fact that our data were not missing completely at random (MCAR, Supplementary Figure 2 and 3), we performed multiple imputation, a procedure that reduces bias at the cost of introducing imprecision. We used chained equations in the mice package in R[20]: systolic and diastolic blood pressure, heart rate, oxygen saturation, respiratory rate, conscious level, age and sex were used to predict all missing variables, generating five imputed datasets.
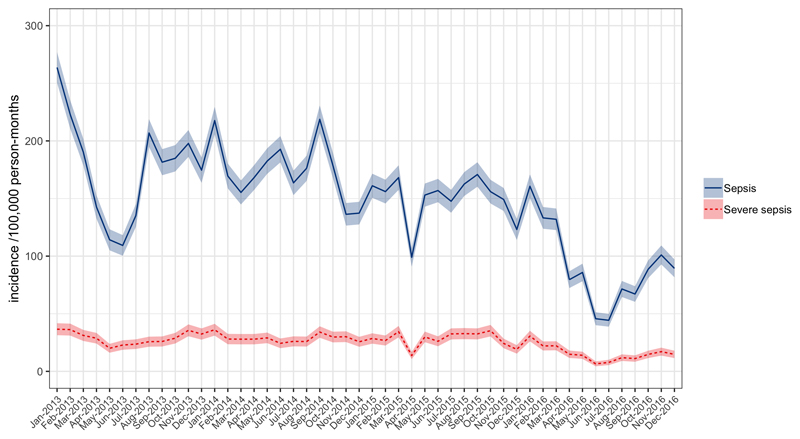
Figure 3.
Population incidence of adult sepsis (blue solid line) and severe sepsis (dotted red line) per 100,000 person-months at risk as a function of time.
To calculate population incidence rates of ED-attending sepsis and severe sepsis we first generated pooled absolute numbers and (assuming a Poisson distribution) variances of cases of sepsis and severe sepsis from the five imputed datasets using Rubin’s rules[21]. To calculate population incidence, we assumed that the calculated population of Blantyre city was at risk and used the pooled variance to construct confidence intervals. Age and period-specific incidences were similarly calculated using the estimated denominators as above. To produce estimates of inpatient sepsis and severe sepsis mortality we pooled mortality estimates and variances from the five imputed datasets. In order to assess for time trends in sepsis incidence we performed negative binomial regression with the a priori covariates year as a continuous variable and rainy season (defined as November-March) as a binary variable. The output of these models were expressed as adjusted incident rate ratios (aIRR) per year increase or for rainy season compared to dry season.
In view of the high proportion of missing data we performed a number of sensitivity analysis. We produced maximum and minimum population incidence estimates by assuming that all missing values were clinically normal or clinically abnormal, respectively, and we produced complete-case incidence estimates by extrapolating from those records that had no missing data by assuming that the proportion of participants with sepsis or severe sepsis was the same in the population with missing data as with complete data. We also explored the effect of differing sepsis definition, defining sepsis as a quick Sequential Organ Failure Assessment[22] (qSOFA) score of 2 or more; a Universal Vital Assessment score[23] of 2-4 and 4 or more; and a Modified Early Warning Score[24] (MEWS) of 5 or greater and calculating incidence using imputed data as above.
All analyses were carried out on R V3.5.1 (R foundation for statistical computing, Vienna, Austria).
Ethical Approval
This study used only anonymized, routinely collected data; it was approved by the Malawi College of Medicine Research Ethics Committee (P.01/18/2331)
Results
From 1st January 2013 to 31st December 2016 there were 217,149 unique adult attendances to the ED recorded in the triage system. There were varying proportions of missing data for these attendances. The majority of variables had < 5% missing data, but three variables had significant proportion of missing data (Supplementary Figure 1): respiratory rate (82% missing), oxygen saturation (65% missing) and conscious level (93% missing). Demographics of the patients in complete-case analysis calculated from the unimputed dataset, are shown in Table 1.
Table 1.
characteristics of included patients from complete-case analysis. HR = heart rate, RR = respiratory rate, SBP = systolic blood pressure, DBP = diastolic blood pressure, SpO2 = oxygen saturation, IQR = interquartile range.
| Non sepsis | Sepsis | Severe Sepsis | ||||
|---|---|---|---|---|---|---|
| Value | n non-missing data | Value | n non-missing data | Value | n non-missing data | |
| Total n | - | 187644 | - | 29505 | - | 3700 |
| Age / years (median, IQR) | 33.2 (25.1-45.4) | 187644 | 33.6 (25.1-45.5) | 29505 | 35.3 (27.3-46.4) | 3700 |
| Male / % | 48% | 187644 | 45% | 29505 | 49% | 3700 |
| Temp. / C (median, IQR) | 36.4 (36-36.9) | 179780 | 35.6 (35.3-38.4) | 29505 | 35.6 (35.3-38.4) | 3700 |
| HR / min (median, IQR) | 88 (77-103) | 182507 | 108 (98-123) | 29367 | 115 (102-130) | 3684 |
| RR / min (median, IQR) | 20 (18-22) | 39718 | 22 (19-25) | 7822 | 22 (19-25) | 1134 |
| SBP / mmHg (median, IQR) | 121 (109-136) | 180731 | 115 (101-131) | 28313 | 84 (77-89) | 3567 |
| DBP / mmHg (median, IQR) | 78 (69-87) | 180742 | 75 (65-86) | 28323 | 57 (49-65) | 3566 |
| SpO2 (median, IQR) | 98% (96-99%) | 64841 | 97% (95-99%) | 11273 | 93 (85-97) | 2001 |
| Alert / % | 97% | 12845 | 97% | 1415 | 80% | 218 |
Population incidence
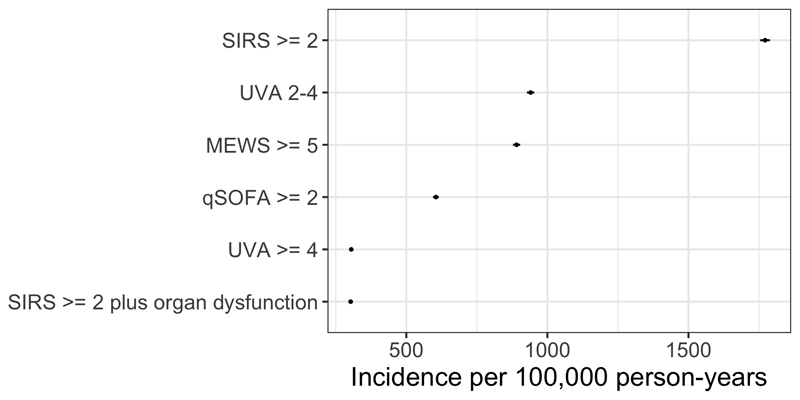
Estimates of adult population incidence rate of sepsis and severe sepsis were 1772 per 100,000 population (95% CI 1754-1789) and 303 per 100,000 population (95% CI 295-310) respectively, following multiple imputation of missing data. Sensitivity analysis assuming that missing data were clinically normal or abnormal gave minimum and maximum estimates (Table 2); differing sepsis definitions gave varying estimates of incidence (Figure 1 and Supplementary Table 1).
Table 2.
Sensitivity analyses showing minimum and maximum bounds of sepsis and severe sepsis incidence assuming all missing values are clinically normal or abnormal, respectively.
| Estimate | Incidence/ 100,000 population |
|---|---|
| Sepsis (minimum) | 1378 |
| Sepsis (complete case) | 1711 |
| Sepsis (maximum) | 2741 |
| Severe sepsis (minimum) | 173 |
| Severe sepsis (complete case) | 239 |
| Severe sepsis (maximum) | 2666 |
Figure 1.
Sensitivity analysis showing estimated sepsis population incidence with varying definitions of sepsis. SIRS = Systemic inflammatory response syndrome; UVA = Universal Vital Assessment; qSOFA = quick sequential organ failure assessment; MEWS – modified early warning score.
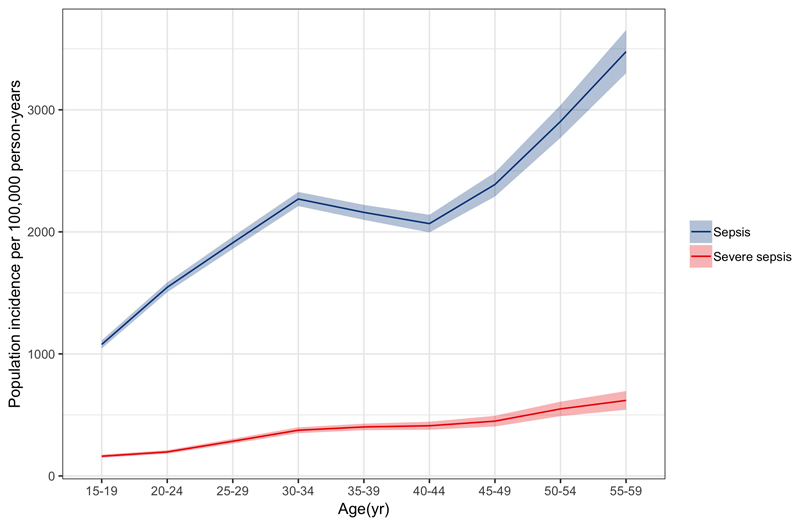
Age stratified population incidence rate showed an increasing incidence with increasing age (Figure 2). The lowest estimates were observed in the 15-19 year group (sepsis 1077 (95% CI 1044-1111) and severe sepsis 162 (95% CI 148-175) cases per 100,000 person years) and highest in 80+ year-olds (sepsis 9726 (95% CI 9075-10377) and severe sepsis 2528 (95% CI 2183-2872) cases per 100,000 person years.
Figure 2.
Age stratified population incidence rate of adult sepsis and severe sepsis for Blantyre, Malawi 2013-16, per 100,000 person-years at risk.
Population incidence of sepsis almost halved over the study period (Figure 3), from 2124 (95% CI 2085 – 2163) sepsis and 348 (95% CI 332 – 364) severe sepsis cases per 100,000 person-years in 2013 to 1099 (95% CI 1072 – 1126) sepsis and 187 (95% CI 176 – 199) severe sepsis cases per 100,000 person years in 2016, with aIRR 0.81 (95% CI 0.78 - 0.84, p < 0.001) per year for sepsis and aIRR 0.84 (95% CI 0.82-0.87, p < 0.001) per year for severe sepsis in multivariable negative binomial regression. There was in addition a suggestion of a seasonal pattern, with incidence higher in wet season (November-March): though this pattern appeared inconsistent year-to-year (Figure 2), multivariable negative binomial regression models found an independent association between rainy season vs dry for sepsis (aIRR 1.11 [95% CI 1.04-1.19, p = 0.002] and severe sepsis (IRR 1.13 [95% CI 1.06-1.21, p < 0.001].
Inpatient outcome
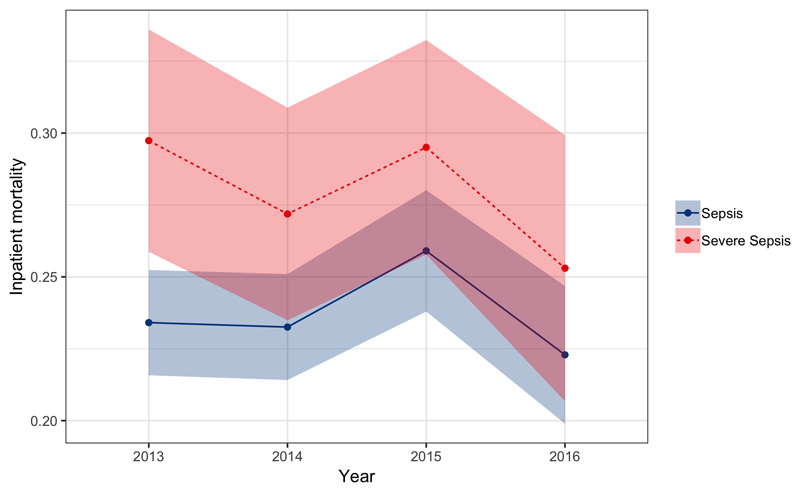
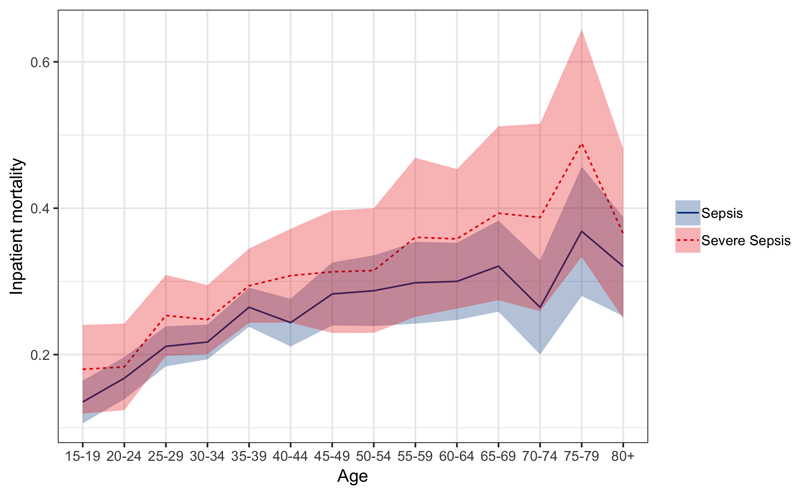
For the period 2013-2016 outcome data were available for 23,787 adult inpatients. Of these, 21,136 (89%) could be matched to a unique record in the ED which provided admission physiology. Of these matched records, 4810 patients died. Pooled inpatient sepsis mortality following imputation of missing data for the entire period was 23.7% (95% CI 22.7-24.7%), severe sepsis mortality was 28.1% (95% CI 26.1 – 30.0%). Despite the reduction in population incidence of sepsis and severe sepsis, there was no apparent mortality trend for sepsis or severe by year with overlapping confidence intervals for mortality rate estimates for all years 2013 – 2016 (Figure 4). Mortality increased with age for both sepsis and severe sepsis, increasing with age up to around age 35 and then more slowly with age thereafter (Figure 5).
Figure 4.
Sepsis (blue solid) and Severe Sepsis (red dotted) inpatient mortality as a function of time. Shaded areas are 95% confidence intervals.
Figure 5.
Sepsis (blue solid) and Severe Sepsis (red dotted) age-stratified inpatient mortality. Shaded areas are 95% confidence intervals
Discussion
We estimate headline population incidence of ED-attending sepsis and severe sepsis to be 1772 per 100,000 population (95% CI 1754-1789) and 303 per 100,000 population (95% CI 295-310) in Blantyre Malawi from 2013-2016. In addition, the population presenting with sepsis is young, with a median age of 34 years (sepsis) or 35 years (severe sepsis).
Estimates of sepsis mortality from high-income settings vary depending on the definitions and coding strategies, making direct comparisons difficult. A recent meta-analysis of 27 studies[2] from seven high-income countries found an incidence rate of 437 (95% CI, 334–571) for sepsis and 270 (95% CI, 176–412) for severe sepsis cases per 100,000 person-years. Hospital mortality was 17% for sepsis and 26% for severe sepsis, but these estimates were largely derived from discharge databases. These may not be directly comparable because diagnoses are often based on classification systems such as ICD-10, rather than admission physiology and patients are largely admitted to hospital (rather than ED attendees, as here). Two studies calculated population incidence of emergency-department treated sepsis/severe sepsis, which may be more comparable, estimating a sepsis incidence of 731 cases per 100,000 person-years in Denmark[25], and a severe sepsis of 140 and 265 per 100,000 person-years in the United States[26] and Denmark[25], respectively. The participants presenting with sepsis in high-income settings in the systematic review had a median age ranging from 47-73, which is considerably higher than we report from Malawi. One study[27] has estimated ITU-treated sepsis incidence in Brazil – a middle income country - and found it to be 290 (95% CI 238-351) adult cases per 100,000 population per year, comparable to our estimate of ED-attending severe sepsis.
It is therefore probable - especially given the paucity of variables that were available in our study to define sepsis and severe sepsis - that the incidence of sepsis in Blantyre, Malawi is higher than in high income settings. In addition, it is occurring in a younger population, and despite this, mortality remains high. The reasons for this are not apparent from our data. HIV may play a role and other prevalent pathogens such as tuberculosis or malaria may also be implicated. HIV is most prevalent in young adults and hence may drive sepsis presentation at a younger age. It could also contribute to the blunting of the usual inpatient relationship between age and mortality that we describe; inpatient sepsis mortality for the 35-40 year old age group was not dramatically different from the 80+ year old age group in this data set, for example: 26.4% (95% CI 23.8- 29.1%) vs 32.0% (95% CI 25.3- 38.8%). In high income settings, mortality in the elderly might be expected to be significantly higher than in young adults: in one large study in the US for example, sepsis mortality was 18.0% in those 80+ versus 8.6% in the 20-39 age group[28]
We saw a clear trend in decline in sepsis incidence over time, with a superimposed seasonal pattern, and no clear change in case fatality rate. Our data provide no insight into the drivers of this phenomenon, although there are several possible explanations. Firstly, this could represent a true change in in sepsis incidence. Significant progress has been made in Malawi with both control of HIV (through antiretroviral provision), and malaria, both of which could contribute to a decrease in sepsis incidence. Indeed, life expectancy at birth in Malawi has increased from 42 years in 2000 to 64 in 2017[29] which is consistent with the significant reduction in incidence in sepsis that we have seen in this study. Alternatively, changes in referral patterns, or coding or data entry practices might introduce artefactual error. We are aware of at least one quality improvement programme at one of the health centres that refer to QECH that occurred during the study period, for example. Despite the apparent reduction in incidence, inpatient mortality remained unchanged, highlighting the fact that sepsis, once established, carries a high mortality and the need for improved quality of care from recognition to management in the Malawian setting. The seasonal pattern we observe is likely related to well recongnised patterns in the causes of febrile illness in Malawi, with higher incidence of invasive Salmonella – an important pathogen in this setting[30] – in the rainy season, for example[11].
There are significant limitations to our study. Most notably, there were significant proportions of missing data. We have used imputation to reduce bias compared to simpler imputation schemes such as assuming clinically normal data for missing values, but this will be at the expense of imprecision. We used a modified Sepsis-2 definition of sepsis[19] using the systemic inflammatory response syndrome (SIRS) criteria, despite the publication of newer (Sepsis-3) definitions. The components of the new definition (the sequential organ dysfunction assessment or SOFA score[1]) were not available in our databases, and indeed are rarely available in routine clinical care in our hospital. Furthermore, Sepsis-2 based incidence estimates provide some “backward compatibility” with previous estimates from high-income settings for comparison. True Sepsis-2 sepsis definitions require a suspicion or confirmation of infection plus SIRS components; these were lacking and we used the presence of fever or hypothermia as a proxy. This might overestimate sepsis incidence, although in clinical practice in Malawi the presence of fever constitutes sufficient evidence to suspect infection. In defining severe sepsis, we were again limited by the data available to us, and used a relatively narrow definition of shock, low oxygen saturations or low conscious level; our estimates of severe sepsis are therefore very likely to be an underestimate. Though we produce population incidence estimates and mortality for sepsis and severe sepsis, these two populations are subtly different: incidence calculations are for ED-attending sepsis/severe sepsis whereas mortality is for patients who have been admitted to the wards. The mortality for ED-attendees is unknown, but would be expected to be lower.
There were other limitations arising from our use of routinely captured data. The databases we used would not capture patients who died in the ED; this could result in an underestimate of sepsis mortality. However, and though our data can not address this question, our experience of the ED at QECH suggests that death in the ED is uncommon. Many patients with sepsis will have been discharged from the ED (either with or against medical advice) and managed as outpatients, If they died at home then they would not be captured in our sepsis mortality estimates; furthermore in the Malawian setting it is likely that some people develop sepsis at home and die without seeking health care. They too would not be captured either in our incidence or mortality estimates. Some participants recorded in the database with apparently unique patient IDs may in fact represent re-registration of the same individual. The databases we used are not linked to investigations performed so HIV status of participants and aetiology of sepsis are unknown.
In conclusion, we have used a large, dataset from a single African centre to calculate, to our knowledge, the first population incidence estimates for sepsis for a country in sSA. We find that sepsis is common, affecting a young population, and despite this, carries a high mortality. Previous estimates of 31 million sepsis cases, 19.4 million severe sepsis cases and 5.3 million deaths are extrapolated from high income settings to low- and middle-income ones, where 83% of the world’s population live[2], and are very likely underestimates. A speculative extrapolation of our findings to all low and middle-income countries suggests that the worldwide incidence could be as high as 111.4 million sepsis cases and 21.4 million severe sepsis cases. This very wide disparity in estimates highlights the problems with extrapolating incidence estimates across very different settings and emphasizes the need for data to guide the public health response to sepsis in sSA and other low-income settings: to describe the basic epidemiology but also develop interventional strategies that can be deployed at scale in low resource settings.
Supplementary Material
Summary.
Sepsis epidemiology in sub-Saharan Africa (sSA) is poorly described. Using large databases from a Malawian teaching hospital, we present some of the first estimates of sepsis population incidence from sSA, and find it to be high compared to high-income settings.
Funding
This article was funded by a DFID/MRC/Wellcome Joint Global Health Trials (MR/P020577/1). Additional funding was received by the National Institute for Health Research using Official Development Assistance (ODA) funding (the African Research Collaboration on Sepsis,17/63/42). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
JL is supported by a Wellcome Trust clinical PhD fellowship (109105z/15/a).
MA is supported by a Wellcome Trust clinical PhD fellowship (203919/Z/16/Z).
Footnotes
Transparency declarations
We have no conflicts of interest to declare.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [cited 2016 Feb 23];JAMA. 2016 315:801. doi: 10.1001/jama.2016.0287. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Am J Respir Crit Care Med. Vol. 193. American Thoracic Society; 2016. [cited 2018 Jul 18]. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations; pp. 259–72. [Internet] [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation. World Malaria Report. Geneva: 2018. [Google Scholar]
- 4.GBD 2016 Lower Respiratory Infections Collaborators C. Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. Lancet Infect Dis. Vol. 18. Elsevier; 2018. [cited 2019 Apr 25]. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016; pp. 1191–210. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2016 Meningitis Collaborators JR. Kassebaum NJ, Blake N, Glennie L, Wright C, Nichols E, et al. Lancet Neurol. Vol. 17. Elsevier; 2018. [cited 2019 Apr 25]. Global, regional, and national burden of meningitis, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016; pp. 1061–82. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2017 Typhoid and Paratyphoid Collaborators JD. Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE, et al. Lancet Infect Dis. Vol. 19. Elsevier; 2019. [cited 2019 Apr 25]. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017; pp. 369–81. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob ST, Banura P, Baeten JM, Moore CC, Meya D, Nakiyingi L, et al. Crit Care Med. Vol. 40. NIH Public Access; 2012. [cited 2015 Nov 2]. The impact of early monitored management on survival in hospitalized adult Ugandan patients with severe sepsis; pp. 2050–8. [Internet]. Available from: /pmc/articles/PMC3378757/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. Jama. 2017;318:1233–40. doi: 10.1001/jama.2017.10913. [Internet]. 2017/10/04. Available from: https://jamanetwork.com/journals/jama/articlepdf/2654854/jama_Andrews_2017_oi_170091.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. Malawi Country Profile. [cited 2019 Feb 28]; [Internet]. Available from: http://aidsinfo.unaids.org/
- 10.Kazembe LN, Kleinschmidt I, Sharp BL. Patterns of malaria-related hospital admissions and mortality among Malawian children: An example of spatial modelling of hospital register data. Malar J. 2006;5 doi: 10.1186/1475-2875-5-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, et al. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. [cited 2019 Oct 25];Clin Infect Dis. 2008 46:963–9. doi: 10.1086/529146. [Internet] [DOI] [PubMed] [Google Scholar]
- 12.Bell M, Archibald LK, Nwanyanwu O, Dobbie H, Tokars J, Kazembe PN, et al. Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. Int J Infect Dis. 2001;5:63–9. doi: 10.1016/s1201-9712(01)90027-x. [Internet] [DOI] [PubMed] [Google Scholar]
- 13.Waitt PI, Mukaka M, Goodson P, SimuKonda FD, Waitt CJ, Feasey N, et al. Sepsis carries a high mortality among hospitalised adults in Malawi in the era of antiretroviral therapy scale-up: a longitudinal cohort study. [cited 2015 Sep 22];J Infect. 2015 70:11–9. doi: 10.1016/j.jinf.2014.07.004. [Internet]. Available from: /pmc/articles/PMC4291151/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feasey NA, Houston A, Mukaka M, Komrower D, Mwalukomo T, Tenthani L, et al. A reduction in adult blood stream infection and case fatality at a large African hospital following antiretroviral therapy roll-out. [cited 2016 Jun 22];PLoS One. 2014 9:e92226. doi: 10.1371/journal.pone.0092226. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wall EC, Mukaka M, Denis B, Mlozowa VS, Msukwa M, Kasambala K, et al. Goal directed therapy for suspected acute bacterial meningitis in adults and adolescents in sub-Saharan Africa. Borrow R, editor. [cited 2019 May 7];PLoS One. 2017 12:e0186687. doi: 10.1371/journal.pone.0186687. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aston SJ, Ho A, Jary H, Huwa J, Mitchell T, Ibitoye S, et al. Aetiology and Risk Factors for Mortality in an Adult Community-Acquired Pneumonia Cohort in Malawi. [cited 2019 May 7];Am J Respir Crit Care Med. 2019 doi: 10.1164/rccm.201807-1333OC. [Internet]. rccm.201807-1333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SanJoaquin MA, Allain TJ, Molyneux ME, Benjamin L, Everett DB, Gadabu O, et al. Surveillance Programme of IN-patients and Epidemiology (SPINE): Implementation of an Electronic Data Collection Tool within a Large Hospital in Malawi. PLoS Med. 2013;10:e1001400. doi: 10.1371/journal.pmed.1001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Statistics Office of Malawi. 2018 Malawi Population and Housing Census. Zomba, Maalwi: [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. [cited 2018 Jul 18];Crit Care Med. 2003 31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [Internet] [DOI] [PubMed] [Google Scholar]
- 20.van Buuren S, Groothuis-Oudshoorn K. mice : Multivariate Imputation by Chained Equations in R . [cited 2019 Apr 25];J Stat Softw. 2011 45:1–67. [Internet]. Available from: http://www.jstatsoft.org/v45/i03/ [Google Scholar]
- 21.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ, USA: John Wiley & Sons, Inc; 1987. [cited 2019 Apr 25]. [Internet] [DOI] [Google Scholar]
- 22.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [cited 2018 Jun 28];JAMA. 2016 315:762. doi: 10.1001/jama.2016.0288. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore CC, Hazard R, Saulters KJ, Ainsworth J, Adakun SA, Amir A, et al. Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub-Saharan Africa. [cited 2018 Jul 18];BMJ Glob Heal. 2017 2:e000344. doi: 10.1136/bmjgh-2017-000344. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbe CP, Kruger M, Rutherford P, Gemmel L. QJM. Vol. 94. Oxford University Press (OUP); 2001. Validation of a modified Early Warning Score in medical admissions; pp. 521–6. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen DP, Laursen CB, Jensen TG, Hallas J, Pedersen C, Lassen AT. Incidence Rate of Community-Acquired Sepsis Among Hospitalized Acute Medical Patients—A Population-Based Survey*. [cited 2019 Apr 25];Crit Care Med. 2015 43:13–21. doi: 10.1097/CCM.0000000000000611. [Internet]. Available from: https://insights.ovid.com/crossref?an=00003246-201501000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Strehlow MC, Emond SD, Shapiro NI, Pelletier AJ, Camargo CA. National Study of Emergency Department Visits for Sepsis, 1992 to 2001. [cited 2019 Apr 25];Ann Emerg Med. 2006 48:326–331.e3. doi: 10.1016/j.annemergmed.2006.05.003. [Internet] [DOI] [PubMed] [Google Scholar]
- 27.Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. [cited 2019 Oct 25];Lancet Infect Dis. 2017 17:1180–9. doi: 10.1016/S1473-3099(17)30322-5. [Internet] [DOI] [PubMed] [Google Scholar]
- 28.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. JAMA. Vol. 318. American Medical Association; 2017. [cited 2019 May 24]. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014; pp. 1241–9. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Bank. Wold Bank: Life Expectancy at Birth. [cited 2019 Apr 25]; [Internet]. Available from: https://data.worldbank.org.
- 30.Musicha P, Cornick JE, Bar-Zeev N, French N, Masesa C, Denis B, et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. [cited 2018 Jun 19];Lancet Infect Dis. 2017 17:1042–52. doi: 10.1016/S1473-3099(17)30394-8. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.