Abstract
Chiral sugar derivatives are potential cyclitol surrogates in the Ca2+-mobilizing intracellular messenger d-myo-inositol 1,4,5-trisphosphate [Ins(1,4,5)P3]. Six novel polyphosphorylated analogues derived from both d- and l-glucose were synthesized. Binding to Ins(1,4,5)P3 receptors [Ins(1,4,5)P3R] and abilities to release Ca2+ from intra-cellular stores via type 1 Ins(1,4,5)P3Rs were investigated. β-d-Glucopyranosyl 1,3,4-trisphosphate, with similar phosphate regiochemistry and stereochemistry to Ins(1,4,5)P3 and α-d-glucopyranosyl 1,3,4-trisphosphate are full agonists, being equipotent and 23-fold less potent than Ins(1,4,5)P3 respectively in Ca2+-release assays, and similar to Ins(1,4,5)P3 and 15-fold weaker in binding assays. They can be viewed as truncated analogues of adenophostin A and refine structure-activity relationship understanding for this Ins(1,4,5)P3R agonist. l-Glucose-derived ligands, methyl α-l-glucopyranoside 2,3,6-trisphosphate and methyl α-l-glucopyranoside 2,4,6-trisphosphate are also active, while their corresponding d-enantiomers, methyl α-d-glucopyranoside 2,3,6-trisphosphate and methyl α-d-glucopyranoside 2,4,6-trisphosphate, are inactive. Interestingly, both l-glucose-derived ligands are partial agonists: they are amongst the least efficacious agonists of Ins(1,4,5)P3R yet identified, providing new leads for antagonist development.
Introduction
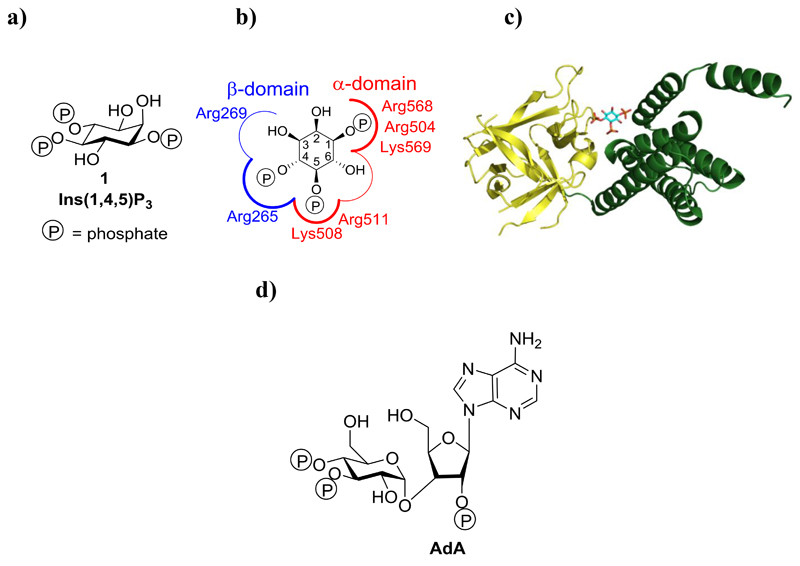
d-myo-inositol 1,4,5-trisphosphate [Ins(1,4,5)P3, 1] is a second messenger that binds to tetrameric d-myo-inositol 1,4,5-trisphosphate receptors [Ins(1,4,5)P3Rs] on the endoplasmic reticulum. Ins(1,4,5)P3Rs are Ca2+ channels that open to release Ca2+ to the cytosol.1,2 The resulting local or global increases in cytosolic Ca2+ concentration regulate diverse cellular processes, including mitochondrial metabolism, cell proliferation, differentiation, smooth muscle contraction, secretion, exocytosis and ion channel opening.3
Ins(1,4,5)P3 (Figure 1a) binds to the Ins(1,4,5)P3-binding core (IBC; residues 224-604) close to the N-terminus of each of the four Ins(1,4,5)P3R subunits (Figure 1b, c). The IBC consists of an α-helical domain and a β-trefoil domain, between which there is a cleft rich in basic amino acid residues.4 Ins(1,4,5)P3 binding within the cleft allows phosphates at positions 1 and 5 to interact with the α-domain, while the 4-phosphate interacts with the β-domain (Figure 1c).5 As Ins(1,4,5)P3 interacts with both domains, it pulls the two sides of the clam-like IBC together.6,7 The clam closure leads to channel opening, possibly by re-arranging Ca2+-binding sites such that Ca2+ can bind to the Ins(1,4,5)P3R and trigger conformational changes that lead to opening of the Ca2+-permeable pore.1,6,7
Figure 1.
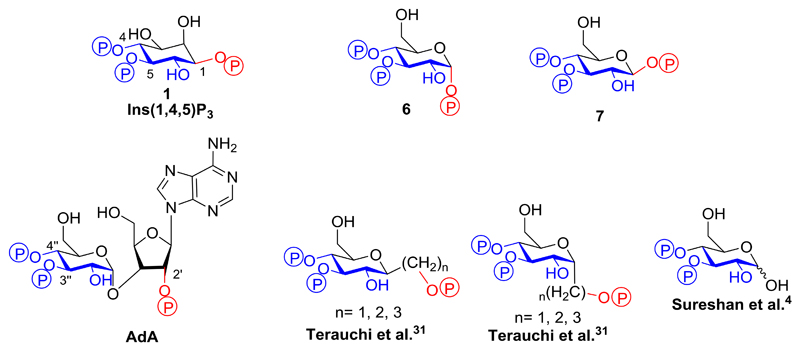
Structures of Ins(1,4,5)P3R and its agonists (a) Structure of Ins(1,4,5)P3 (1). (b) Binding mode of 1 to the IBC of Ins(1,4,5)P3R with key amino acid residues involved in binding labelled. (c) Crystal structure of the IBC of type 1 InsP3R with Ins(1,4,5)P3 (1) bound (PDB: 1N4K). The α-domain is shown in green and the β-domain in yellow. (d) Structure of the Ins(1,4,5)P3R agonist adenophostin A (AdA).
Modulators of Ins(1,4,5)P3R activity are highly sought after, and many studies have examined structure-activity-relationship (SARs) of ligands binding to Ins(1,4,5)P3R attempting to identify partial agonists or antagonists.8–10 Synthetic analogues have played a key role in this process, including the potent glyconucleotide adenophostins5,11 (Figure 1d), with a recent synthetic study reporting the effects of replacing the glucose moiety of adenophostin A (AdA) with a chiro-inositol core.12 This highlights developing interest in examining less explored isomers of inositol than the myo-form.13
The phosphates attached to the 4- and 5-positions of Ins(1,4,5)P3 (Figure 1a) are thought to be essential to agonist activity as each interacts with a different domain of the IBC. 4,14 The 1-phosphate increases affinity, but it is not essential for receptor activation.5 The hydroxyl group attached to the 6-position of Ins(1,4,5)P3 appears to be important for optimal activity but it is not essential,15 while hydroxyl groups attached to the 2- and 3-positions are less involved in ligand binding.16
A few Ins(1,4,5)P3R antagonists have been identified, but these suffer major drawbacks including poor target selectivity, cell impermeablility (heparin),17 inconsistent effectiveness in assays (2-aminoethoxydipheylborane, 2-APB),17 low potency (caffeine),17,18 and disputed activity (xestospongins).17 For decades, analogues of Ins(1,4,5)P3 have been synthesized and investigated in attempts to discover a selective antagonist for Ins(1,4,5)P3R that could be made, at least temporarily, cell-permeable with enzymatic- or photo-labile protecting groups.19,20 Very recently, there has also been work carried out by Li et al. that describes the delivery of inositol phosphates into cells via lysosomes rendering phosphate protecting groups unecessary.21 To date, however, only a small number of analogues of Ins(1,4,5)P3 with minor structural modifications have been identified as partial agonists or antagonists at Ins(1,4,5)P3R. Most of these compounds demonstrate that conservative modifications to the phosphates attached to positions 4 and 5 and the hydroxyl group attached to position 3 can lead to degrees of antagonist activity. 22–25 However, many analogues of Ins(1,4,5)P3 with modifications to the same regions are inactive.26 Attaching a bulky substituent to the axial 2-position hydroxyl can also lead to partial agonist activity27 and interestingly even using a simple benzene ring as a surrogate for inositol in a benzene polyphosphate approach, as a dimer or biphenyl, can provide low-affinity antagonists.28,29
Stimulated in part by the discovery of the adenophostins,5,10,30 there have been a number of studies to investigate polyphosphates of d-glucose and of other sugars,30–34 as inositol phosphate analogues (see below).35 By using such carbohydrates, the need for optical resolution of protected cyclitol precursors or resulting phosphate regioisomers is bypassed, as chiral starting materials are readily available. Also, the structural features of carbohydrates offer additional opportunities for synthetic versatility. In this study, we return to and expand upon, the use of d-glucose to try to identify novel Ins(1,4,5)P3R ligands. We also investigate, perhaps counter-intuitively, the use of l-glucose as a starting material.
Although several studies have generated d-glucose-based ligands of Ins(1,4,5)P3R,30,31,34 no ligands based on the l-glucose enantiomer have been synthesized and nor is it known whether ligands with this scaffold would bind to Ins(1,4,5)P3R. In a previous study,5 Ins(4,5)P2 [albeit a low-affinity Ins(1,4,5)P3R ligand] was effectively mimicked by a d-Gluc(3,4)P2 surrogate. We noted that both d- and l-glucose offer three hydroxyl groups of the requisite relative configuration that could in principle be used to mimic the 4,5,6-hydroxyl groups in myo-inositol. Thus, we anticipated that we could use this similarity (Figure 2, highlighted in red), alongside intrinsic structural differences of l- and d-glucose to prepare diverse chiral ligands with appropriately located phosphates These would present different structural motifs to the Ins(1,4,5)P3R and allow further investigation of the binding site and perhaps identify novel activity. With this in mind we designed and synthesized six novel ligands based on l-glucose and d-glucose (Figure 3) and evaluated their activity at Ins(1,4,5)P3R.
Figure 2.
Structures of methyl α-d-glucopyranoside and methyl α-l-glucopyranoside relative to myo-inositol with the shared stereochemistry of the hydroxyl groups highlighted in red.
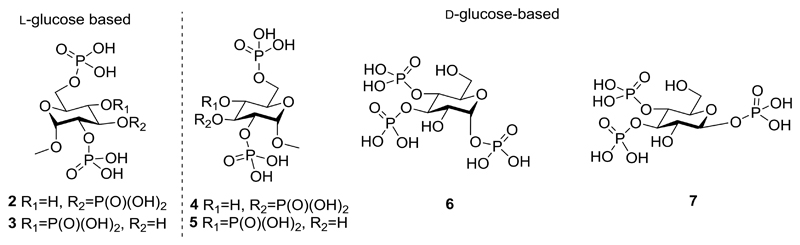
Figure 3.
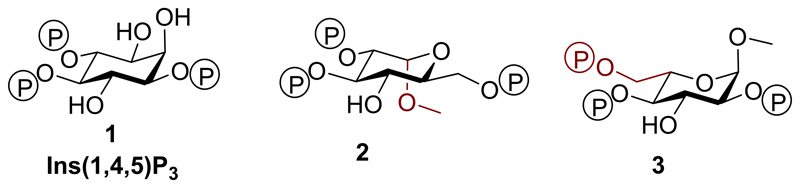
Structures of Ins(1,4,5)P3 (1) analogues: methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) and methyl α-l-glucopyranoside 2,4,6-trisphosphate (3), methyl α-d-glucopyranoside 2,3,6-trisphosphate (4) and methyl α-d-glucopyranoside 2,4,6-trisphosphate (5), α-d-glucopyranosyl 1,3,4-trisphosphate (6), β-d-glucopyranosyl 1,3,4-trisphosphate (7).
We ensured that the designed ligands retained structures equivalent to the critical 4,5-bisphosphate motif of Ins(1,4,5)P3 and had no major structural modifications in regions believed to be necessary for ligand binding; modifications in regions equivalent to the 2-O-position were permitted, as these were expected to remain outside the binding pocket. We hypothesized that the l-glucose-based ligands [methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) and methyl α-l-glucopyranoside 2,4,6-trisphosphate (3)] might have sufficiently conservative structural changes relative to Ins(1,4,5)P3 that they could still bind to Ins(1,4,5)P3R, possibly with novel activity. The d-glucose-based ligands [methyl α-d-glucopyranoside 2,3,6-trisphosphate (4) and methyl α-d-glucopyranoside 2,4,6-trisphosphate (5)] were not expected to adopt orientations that position the phosphate groups in appropriate regions of the IBC (see Supporting Information for details of all possible predicted binding modes). Their bioassay was designed to enable confirmation of enantioselectivity. From a practical standpoint, the synthesis of ligands 4 and 5 was optimized first with d-glucose before the commercially available, but considerably more costly, l-glucose was used to make the respective l-enantiomers, 2 and 3. We anticipated that the two ligands with phosphates at the anomeric carbon, α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7), would help to elucidate the basis for the high affinity of AdA.5,12,36 We expected both novel truncated analogues to be agonists due to their structural similarity to Ins(1,4,5)P3, but were interested to compare their activities with AdA and the previously analysed truncated analogue, Glu(3,4)P2.5
Results
Chemistry
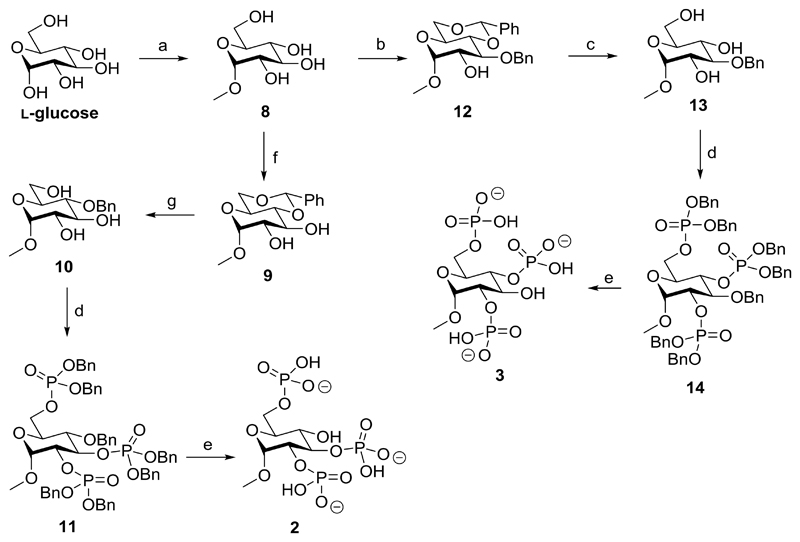
Methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) was synthesized in a five-step route from readily available l-glucose (Scheme 1). Refluxing l-glucose in an acidic methanol solution resulted in protection of the anomeric hydroxyl with a methyl group. Multiple recrystallizations from ethanol afforded methyl α-l-glucopyranoside 8 in 45% yield. The 4- and 6-position hydroxyls were protected with a benzylidene group to form 9 in 91% yield. This benzylidene group was reduced regioselectively, opening to form methyl 4-O-benzyl-α-d-glucopyranoside (10). The benzylidene reduction was first attempted with borane-THF and AlCl3, but this was found to be insufficiently regioselective and produced inseparable regioisomers. The reaction was successfully carried out with borane-THF and La(Tf)3 following the method of Shie et al.37 to yield 10 in 31% yield following purification. The hydroxyl groups in triol 10 were then phosphitylated with dibenzyl diisopropylphosphoramidite and subsequent oxidation with mCPBA formed 11 in 86% yield. The benzyl protecting groups on phosphates and O-4 were then removed by stirring a solution of 11 with Pearlman’s catalyst under hydrogen overnight. After filtration to remove catalyst and evaporation of the solvent, 2 was collected as the triethylammonium salt in 53% yield.
Scheme 1.
Synthesis of l-glucose-derived ligands: methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) and methyl α-l-glucopyranoside 2,4,6-trisphosphate (3)a
aReagents and conditions: (a) MeOH, reflux, 5d (b) (1) TMSCl, py, 22h (2) DCM, benzaldehyde, FeCl2·6H2O, MeCN, triethylsilane, 0°C - room temperature, 1.5h (c) MeOH, H2O, 1M HCl(aq), reflux, 3h (d) (1) DCM, 5-phenyl-1H-tetrazole, (BnO)2PN(iPr)2, 20h (2) mCPBA, -78°C - room temperature (e) MeOH:H2O (10:1 v/v), cat. Pd(OH)2/C, H2, 24h (f) MeCN, benzaldehyde dimethyl acetal, cat. CSA, 24h (g) BH3-THF, La(Tf)3, 7d.
Methyl α-l-glucopyranoside 2,4,6-trisphosphate (3) was also synthesized in five steps from l-glucose, diverging from the synthesis of 2 after the initial methylation step. Methyl α-l-glucopyranoside (8) was protected in a regioselective one-pot reaction that involved persilylation followed by FeCl2-catalyzed benzylidene protection as described by Bourdreux et al.38 to yield 12 in 79% yield. The acid-labile benzylidene group was removed through reflux with HCl(aq) to form 13 in 93% yield. Phosphorylation using standard phosphoramidite methodology39 was then employed to give 14 in 40% yield. After debenzylation with hydrogen and Pearlman’s catalyst and filtration and evaporation of the solvent, the final product, 3, was collected as its triethylammonium salt in 90% yield.
Both α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7) were synthesized via a divergent route, starting with allyl 2,6-di-O-Bn-α-d-glucopyranoside (15).34 Palladium chloride-catalyzed deallylation yielded 16, although purification of this compound was found to be very difficult at this step and purification after phosphorylation proved to be much more effective. Thus, slightly impure 16, was phosphorylated to yield a pure, partially separable mixture of the epimeric phosphates 17 and 18 (56% yield total: 14% 17, 19% 18, 23% mixed epimers). It was unclear how stable 17 and especially the phosphorylated β-epimer 18 would be, as there are reports of compounds with phosphate groups at the anomeric carbon atom being unable to survive purification by silica column chromatography in some cases and not in others.40–42
We found in this case that both compounds survived silica gel chromatography, although 18 showed slight degradation by 31PNMR over time (approx. 30%. after three weeks at 4°C). Catalytic hydrogenolysis of both compounds 18 and 19 was carried out in the presence of sodium bicarbonate to prevent acidic hydrolysis of the potentially labile C-1 phosphates. The final products 6 and 7 were purified by reverse phase ion pair chromatography and collected as their triethylammonium salts.
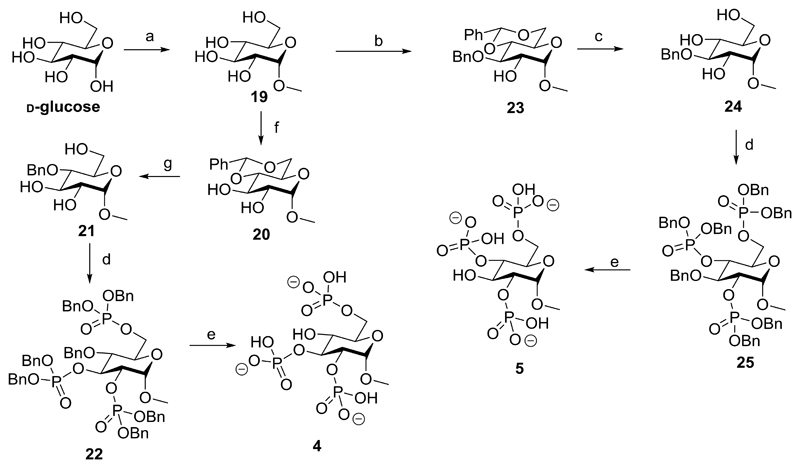
Methyl α-d-glucopyranoside 2,3,6-trisphosphate (4) and methyl α-d-glucopyranoside 2,4,6-trisphosphate (5) were synthesized using the same methods as described for their enantiomers (1 and 2) starting the route with d-glucose (Scheme 3).
Scheme 3.
Synthesis of d-glucose-derived ligands: methyl α-d-glucopyranoside 2,3,6-trisphosphate (5) and methyl α-d-glucopyranoside 2,4,6-trisphosphate (4)a
aReagents and conditions: (a) MeOH, reflux, 5d (b) (1) TMSCl, py, 22h (2) DCM, benzaldehyde, Fe(II)Cl2·6H2O, MeCN, triethylsilane, 0°C - room temperature, 1.5h (c) MeOH, H2O, 1M HCl (aq), reflux, 3h (d) (1) DCM, 5-phenyl-1H-tetrazole, (BnO)2PN(iPr)2, 20h (2) mCPBA, - 78°C - room temperature (e) MeOH:H2O (10:1 v/v), cat. Pd(OH)2/C, H2, 24h (f) MeCN, benzaldehyde dimethyl acetal, cat. CSA, 24h (g) BH3-THF, La(Tf)3, 7d.
The relative stabilities of α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7) were first investigated by allowing each compound (as triethylammonium salts) to remain in a solution of D2O at room temperature at pH 7. Over the course of two months, neither isomer showed any sign of degradation. Following this, a mixture of the compounds in a known starting ratio was exposed to increasingly harsh conditions and relative isomer degradation was monitored through 1H NMR spectroscopy (see Supporting Information Figs. S5 and 6). From the results of the hydrolysis study, we determined that the β-epimer (7) degraded more readily than the corresponding α-epimer (6). Both compounds were however surprisingly durable and required strongly acidic conditions to be fully hydrolyzed (ie at pH1 for 1d), while strongly basic conditions (ie pH 14 for 1d) only produced limited degradation (and at pH 10 for a week there was no change). We are therefore confident that both compounds remained intact during the near-neutral conditions of the biological assays. All final compounds were assessed by HPLC for purity (Fig. S6) and products from acid hydrolysis were examined by HPLC using a synthetic standard of D-glucose 3,4-bisphosphate5 to confirm the expected hydrolysis product of both compounds (see Supporting Information Fig. S7).
Biology
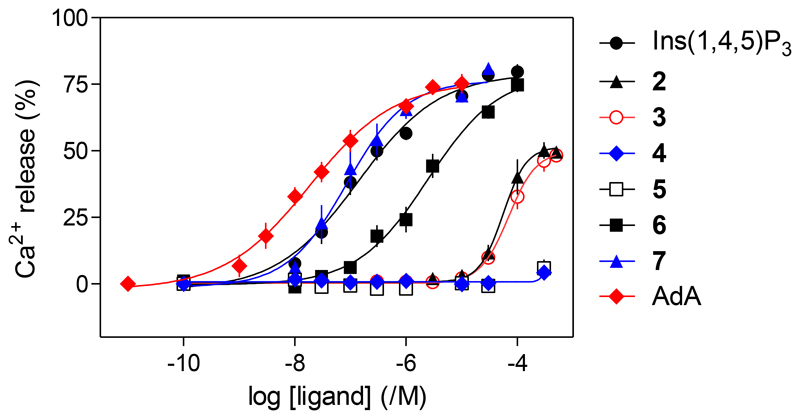
Permeabilized HEK-Ins(1,4,5)P3R1 cells were used to determine the ability of compounds 2-7 (with Ins(1,4,5)P3 and AdA as controls) to evoke Ca2+ release from intracellular stores (Figure 4 and Table). Maximally effective concentrations of Ins(1,4,5)P3, α-d-glucopyranosyl 1,3,4-trisphosphate (6) or β-d-glucopyranosyl 1,3,4-trisphosphate (7) released the same fraction (ca. 80%) of the intracellular Ca2+stores, suggesting that these two epimeric compounds are both full agonists (Figure 4). Compound 7 was equipotent with Ins(1,4,5)P3 and 6 was ca. 20-times less potent than Ins(1,4,5)P3.
Figure 4.
Concentration-dependent effects of Ins(1,4,5)P3 and related ligands on Ca2+release from intracellular stores of permeabilized HEK-Ins(1,4,5)P3R1 cells. Results are mean ± SEM from 5-11 independent experiments, each with duplicate determinations.
Table 1. Receptor binding and Ca2+ Release by Ins(1,4,5)P3R mediated by Ins(1,4,5)P3, AdA and compounds 2-7a.
| Ca2+ release | Binding | ||||||
|---|---|---|---|---|---|---|---|
| pEC50 EC50 | Release (%) | h | pKd Kd (nM) | h | EC50/Kd | ^EC39/Kd | |
| Ins(1,4,5)P3 | 6.90 ± 0.12 126nM |
78.8 ± 1.3 | 0.7 ± 0.1 | 8.06 ± 0.03 8.7 | 1.1 ± 0.2 |
14 (5 - 46) |
17 (5 - 63) |
| 2 | 4.06 ±
0.09* 87.7μM |
56.2 ± 2.6* | 1.4 ± 0.2 | 5.91 ± 0.03* 1230 |
0.9 ± 0.1 |
71 (34 – 148) |
132 (49 – 355) |
| 3 | 3.98 ± 0.04* 104μM |
53.1 ± 5.0* | 1.3 ± 0.2 | 6.26 ± 0.07* 549 |
0.9 ± 0.1 |
191* (123 – 295) |
462* (128 – 1667) |
| 4 | ND | 4.3 ± 2.1# | ND | 48 ± 12$ | ND | ND | |
| 5 | ND | 5.9 ± 2.1# | ND | 53 ± 3$ | ND | ND | |
| 6 | 5.53 ±
0.20* 2.96μM |
78.8 ± 3.0 | 0.9 ± 0.2 | 6.89 ± 0.09* 129 |
0.7 ± 0.1 |
23 (5 - 105) |
|
| 7 | 7.09 ± 0.18 80nM |
75.2 ± 1.4 | 1.0 ± 0.1 | 7.95 ± 0.05 11.2 |
1.1 ± 0.2 |
7 (2 - 29) |
|
| AdA | 7.62 ± 0.12* 24nM |
77.8 ± 4.5 | 0.8 ± 0.1 | 8.86 ± 0.14* 1.4 |
1.2 ± 0.2 |
17 (5 – 51) |
|
Effects of ligands on Ca2+ release from the intracellular stores of permeabilized HEK-Ins(1,4,5)P3R1 cells and on [3H]-Ins(1,4,5)P3 binding to cerebellar membranes are summarized. Results from functional assays are means ± SEM (pEC50 (-log of the half-maximally effective concentration), Ca2+ release (%) and Hill coefficient, h) and means (EC50) from 5-11 independent experiments, each performed in duplicate. Results from binding experiments are means ± SEM (pKd (-log of the equilibrium dissociation constant) and h) and means (Kd) from 3 independent experiments. The pKd values for Ins(1,4,5)P3 and AdA have been published (Mills et al.)43 and are reproduced with permission. Final columns show EC50/Kd or (for partial agonists and Ins(1,4,5)P3) EC39/Kd (mean and 95% CI). ND, not determined. *P < 0.05 relative to Ins(1,4,5)P3.
Ca2+ release evoked by 300 μM of the ligand
Specific binding of [3H]-Ins(1,4,5)P3 in presence of 30μM of competing ligand
EC39 reports the concentration of ligand required to evoke the same Ca2+ release (39% of the intracellular stores) as evoked by a half- maximally effective concentration of Ins(1,4,5)P3.
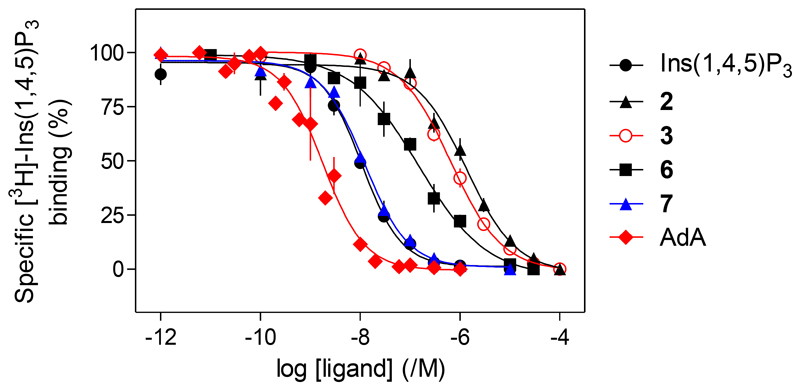
The l-glucose-based ligands methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) and methyl α-l-glucopyranoside 2,4,6-trisphosphate (3) were much less potent than Ins(1,4,5)P3 (Figure 4), while their enantiomers methyl α-d-glucopyranoside 2,3,6-trisphosphate (4) and methyl α-d-glucopyranoside 2,4,6-trisphosphate (5) were, as predicted, inactive. The Ca2+ release evoked by maximally effective concentrations of 2 or 3 was only ca. 70% of that evoked by Ins(1,4,5)P3, suggesting that 2 and 3 are partial agonists. Since partial agonists bind to Ins(1,4,5)P3Rs, but activate them less effectively than full agonists, a partial agonist must bind to more Ins(1,4,5)P3Rs than a full agonist to evoke comparable Ca2+ release. We performed equilibrium competition binding assays using [3H]-Ins(1,4,5)P3 and the active ligands to examine relationships between ligand binding and functional responses. The affinities of 6 and 7 for Ins(1,4,5)P3R aligned with their potencies in functional assays, with 7 having an affinity indistinguishable from that of Ins(1,4,5)P3, while 6 had ca. 15-fold lower affinity (Figure 5, Table). The EC50/Kd values for Ins(1,4,5)P3, AdA, 6 and 7 were similar, consistent with each being a full agonist (Table). Comparison of the concentrations of 2 and 3 required to occupy 50% of binding sites (Kd) and to evoke release of 39% of the Ca2+ stores (EC39, i.e. the Ca2+ release evoked by a half-maximally effective Ins(1,4,5)P3 concentration) confirmed that 2 and 3 are weak partial agonists: their EC39/Kd values (132 and 462, respectively) were much greater than that of Ins(1,4,5)P3 (17). HPLC was used to confirm the purity of the compounds used in the biological assays (details in the Supporting Information).
Figure 5.
Equilibrium-competition binding to cerebellar membranes using [3H]-Ins(1,4,5)P3 (1.5nM) and the indicated concentrations of competing ligands. Results are means ± SEM from 3 independent experiments. The results for Ins(1,4,5)P3 and AdA have been published (Mills et al.)43.
Discussion
Of the glucose polyphosphates considered in this study, the two that bound to Ins(1,4,5)P3R with highest affinity were α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7). Both compounds, which can be considered as truncated analogues of adenophostin A (AdA, Figure 6),44 were found to be full agonists of Ins(1,4,5)P3R, and the β-epimer (7) was equipotent with Ins(1,4,5)P3.
Figure 6.
Structural comparison of Ins(1,4,5)P3 (1), AdA and some of its truncated analogues including α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7). The conserved regions of the structures involved in binding are drawn in blue while the differing auxiliary phosphate is shown in red.
Compounds containing a phosphate group attached to the anomeric carbon atom, as featured in 6 and 7, have not been investigated as Ins(1,4,5)P3R ligands, presumably due to concerns over their stability, at least in the case of the β-epimer.31 Nevertheless, we found that both α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7) were surprisingly durable as their triethylammonium salts and neither compound showed signs of degradation after two months in neutral aqueous solution at room temperature (see Supporting Information for details). Both 6 and 7 were eventually degraded under strongly acidic conditions, and HPLC traces were taken to confirm their hydrolysis to glucose 3,4-bisphosphate (details in the Supporting Information).
Previous studies using synthetic analogues of AdA30–34,45 demonstrated that the adenine moiety significantly increases potency of the agonist, the vicinal phosphates are crucial to activity, and minor adjustments to the placement of the auxiliary phosphate can be tolerated.5,34 The general consensus for AdA binding has been that the ligand interacts with the binding site of Ins(1,4,5)P3R with the 3″, 4″ and 2′ phosphates mimicking the 4, 5 and 1 phosphates of Ins(1,4,5)P3 respectively.27,46 However, these previous studies employed analogues which differed from Ins(1,4,5)P3 in several ways and it has therefore been difficult to isolate the specific impact of replacing the myo-inositol ring with d-glucopyranose.
This has implications for the possible mode of action of AdA and related compounds. Indeed, a cryo-EM study47 of tetrameric Ins(1,4,5)P3R1 has recently proposed that AdA interacts with the IBC in a completely different way to Ins(1,4,5)P3, with the two domains of the IBC being pulled together by the 3″- and 4″-phosphate groups of AdA interacting with one domain and the adenine moiety interacting with the other.47 In this model of AdA binding to Ins(1,4,5)P3R, the glucose bisphosphate structure of AdA only coincidentally resembles the myo-inositol 4,5-bisphosphate of Ins(1,4,5)P3 and there is no structural correspondence between the glucose ring of AdA and the inositol ring of Ins(1,4,5)P3. However, this conclusion does not support the observed activities of the compounds in this study and other AdA analogues; it should therefore be viewed with caution.12,47
In the present study, we found that the closest possible glucose-containing analogue of Ins(1,4,5)P3, namely compound 7, is effectively indistinguishable from Ins(1,4,5)P3 in our assays of Ins(1,4,5)P3R binding and Ca2+ release. This is entirely consistent with the idea that, in the Ins(1,4,5)P3-binding site, the glucopyranoside ring of 7 is closely analogous to the myo-inositol ring of Ins(1,4,5)P3. In turn, this establishes that the flexible Ins(1,4,5)P3-binding site can accommodate the equatorial glucopyranoside hydroxymethyl (CH2OH) group and pyranoside ring oxygen in place of the myo-inositol 3-OH group and C-2, respectively, with no impact on activity. The α-epimer 6 is approximately 27-fold less potent than β-epimer 7 in Ca2+ release. This shows that the axial phosphate group in 6 can still contribute to binding [Gluc(3,4)P2 is much less potent] and is also consistent with an earlier report that d-chiro-Ins(1,3,4)P3, the C-1 epimer of Ins(1,4,5)P3 having an axial 1-phosphate group, had 25-fold lower potency than Ins(1,4,5)P3.35,48
Thus, the effects of trisphosphates 6 and 7 provide strong support for the argument that compounds containing the d-glucopyranosyl 3,4-bisphosphate structure mimic Ins(1,4,5)P3 due to the direct structural analogy between glucopyranosyl and myo-inositol rings depicted in Figures 2 and 6. In such compounds, it is highly likely that the glucose 3,4-bisphosphate structure simply pulls together the two domains of the IBC in the manner proposed for the inositol 4,5-bisphosphate motif of Ins(1,4,5)P3. We recently reported studies in which the glucose ring of AdA12 and ribophostin43 was replaced by d-chiro-inositol, leading to both modest and significant increases in biological activity respectively. It therefore remains to be conclusively established whether the additional components present in the AdA molecule can, counter-intuitively, induce a completely unrelated role for the glucose bisphosphate component of AdA itself, as suggested in the cryo-EM study.47
Previous studies of C-glycosidic truncated analogues of AdA with different chain lengths tethering the third, auxiliary phosphate group (Figure 6) have demonstrated that positioning of this phosphate has a significant effect on the affinity of the agonist.30–34 It has been observed that the Ca2+-releasing potency of these analogues at Ins(1,4,5)P3R decreases as the length and flexibility of the linkage to the auxiliary phosphate increases.34 The orientation of the linkage also plays a role, with axial linkages usually resulting in more potent compounds. However, even the most potent of these compounds [Figure 6, Terauchi et al.31 axial linkage, n = 2] is still weaker than Ins(1,4,5)P3 and compound 7.
Methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) was found to be a partial agonist of Ins(1,4,5)P3R1, while its d-glucose-based enantiomer, compound 4, was inactive. This supports the structural alignment of trisphosphate 2 with Ins(1,4,5)P3 shown in Figure 7 and in our molecular modeling in Figure S3. No such alignment is possible for compound 4 because it does not possess a vicinal bisphosphate motif whose stereochemistry matches that of Ins(1,4,5)P3.
Figure 7.
Ins(1,4,5)P3 (1) and analogues methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) and methyl α-l-glucopyranoside 2,4,6-trisphosphate (3) with their structural differences contribute to their Ins(1,4,5)P3R partial agonist activity in dark red.
In the predicted binding conformation of methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) (Figure S3), the axial methyl group is positioned in a region of the binding site normally occupied by the 3-hydroxyl of Ins(1,4,5)P3. In the design of 2, we further anticipated that the phosphate group at C-6 of l-glucose would mimic the auxiliary 1-phosphate of Ins(1,4,5)P3 to some extent as there is evidence from previous studies showing that the Ins(1,4,5)P3R can accommodate more sterically demanding groups in this region of the binding site.5,49–51 A very recent example of this is that replacement of the Ins(1,4,5)P3 1-phosphate by a pyrophosphate, which increases both charge and steric bulk, does not affect activity.37 In addition, trisphosphate 2 contains an hydroxyl group appropriately placed to mimic the important 6-OH group of Ins(1,4,5)P3.
In studies of Ins(1,4,5)P3 analogues as partial agonists, it has been shown that perturbations in the equivalent of the 3-hydroxyl of Ins(1,4,5)P3 can result in partial agonist activity,52 especially when this disruption (often by means of stereochemical inversion) occurs in conjunction with a modification to the vicinal phosphate pair or other region of the ligand.18,22,25,52 It has been observed in multiple studies that limited, equatorial extension of substituents from the 3 position equivalent can be tolerated,53–55 but larger groups hinder binding56,57 and inversion of the 3-hydroxyl to axial results in a slight decrease in ligand activity.22,58–60 Figure 7 therefore suggests that the axial O-methyl group is the most likely component of 2 that causes it to display partial agonist activity, perhaps by interfering with the ligand binding to the β-domain of the IBC or by reducing the extent of domain closure.
Methyl α-l-glucopyranoside 2,4,6-trisphosphate (3) was designed to bind to the Ins(1,4,5)P3R in a manner that would potentially satisfy the essential binding requirements by positioning the pyranoside ring oxygen in place of the non-essential 3-OH group of Ins(1,4,5)P3 while the axial 1-methoxy group occupied the place of the unimportant 2-OH group of Ins(1,4,5)P3 (Figure 7 and S4). In this binding mode, the l-glucose 6-phosphate group would enter the region of the binding site usually occupied by the 4-phosphate of Ins(1,4,5)P3.
In previous studies, it has been shown that conservative modifications to the phosphates attached to the 4 and 5 equivalent positions (and sometimes in conjunction with a modification to the 3 position equivalent) can produce partial agonists and even low-affinity antagonists.22–25,52,56,61 Bello et al.26 hypothesized that if a ligand could bind to only one side of the IBC (through disruption of the interactions of either the 4 or 5 equivalent phosphates), it would be unable to pull the clam-like structure of the binding site closed and would therefore be unable to activate the receptor. Thus, a suitable modification to the 4-phosphate of Ins(1,4,5)P3 might weaken the important interaction with the β-domain of the clam-shell structure and induce sub-optimal activation of Ins(1,4,5)P3R. However previous studies attempting to generate partial agonists with modifications solely to the 4-phosphate have failed to identify any active Ins(1,4,5)P3 analogues.26
Pleasingly, our assays show that l-glucose trisphosphate 3 also behaves as a partial agonist of Ins(1,4,5)P3R1, with improved binding affinity and higher EC50/Kd ratio than partial agonist 2. The fact that the d-enantiomer 5 is inactive supports the structural alignment of 3 with Ins(1,4,5)P3 depicted in Figure 7. The ″extended″ 4-phosphate group equivalent in 3 may thus disrupt the interaction of the ligand with the β-domain of the IBC as theorized.26 It is likely that the absence in 3 of an equivalent to the 3-OH group in Ins(1,4,5)P3 also contributes to a decreased interaction between the β-domain of the IBC and the ligand. Indeed, activity for 3-deoxy-Ins(1,4,5)P3 at Ins(1,4,5)P3R has been reported to drop to up to 40 fold.10
The two partial agonists, α-l-glucopyranoside 2,3,6-trisphosphate (2) and α-l-glucopyranoside 2,4,6-trisphosphate (3), indicate that perturbations of the ring structure of the ligand are sufficient to induce partial agonism. Both ligands suffer from low affinity and as a result, structurally related compounds are currently being developed that will incorporate similar structural differences to Ins(1,4,5)P3 and hopefully maintain the desired decreased efficacy while increasing affinity. The most promising avenue seems to be adapting the structure of 3 by generating other ligands with extended 4-position phosphate equivalents. This could hypothetically be continued with l-glucose, but inositol could also prove to be a useful starting material as ligands could be synthesized with a similar extension of the 4-position hydroxyl without loss of an equivalent hydroxyl to position 3 in Ins(1,4,5)P3, perhaps thereby improving ligand affinity while retaining partial agonist activity. Such work is in progress.
Conclusions
We have synthesized four novel active ligands for the Ins(1,4,5)P3R based on both d-glucose and l-glucose templates as inositol surrogates. The two ligands based on l-glucose, namely methyl α-l-glucopyranoside 2,3,6-trisphosphate (2) and methyl α-l-glucopyranoside 2,4,6-trisphosphate (3), are low-affinity, low-efficacy partial agonists of Ins(1,4,5)P3R, while their respective d-glucose-based enantiomers 4 and 5 are inactive. Two further synthetic d-glucose-based trisphosphates, α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7) can be regarded as close analogues of Ins(1,4,5)P3 but they are also related structurally to the naturally-occurring glyconucleotide Ins(1,4,5)P3R agonist adenophostin A (AdA). They can therefore further our understanding of how AdA binds to Ins(1,4,5)P3R. Both 6 and 7 were found to be full agonists of Ins(1,4,5)P3R, with the perhaps surprisingly stable β-epimer 7 being equipotent to Ins(1,4,5)P3 itself and potentially useful as a chemical biology tool under physiological conditions (with degradation induced under extremes of pH). The potency of 7 demonstrates that the structural differences between myo-inositol and d-glucose need not result in any decrease in ligand activity. This is consistent with the d-glucopyranosyl 3,4-bisphosphate moiety of AdA directly mimicking the d-myo-inositol 4,5-bisphosphosphate structure of Ins(1,4,5)P3 at the binding site of Ins(1,4,5)P3Rs.
Partial agonists 2 and 3are the first l-glucose-derived ligands that have been synthesized for Ins(1,4,5)P3R. Both compounds provide evidence for the viability of generating partial agonists and potential antagonists of Ins(1,4,5)P3R by deliberately disrupting the crucial moieties involved in binding to the IBC clam shell and pulling the domains together upon ligand binding. We hypothesize that the axial O-methyl group of compound 2 and the extended phosphate in the equivalent of the 4-position phosphate in Ins(1,4,5)P3 of compound 3 cause the partial agonist activity of these compounds by either disrupting the interactions of the ligand with the β domain of the IBC or by preventing complete closure of the IBC upon binding. These partial agonists could prove to be interesting starting points to generate structurally similar compounds with even lower efficacy and higher affinity that could result in the generation of improved partial agonists or antagonists.
Experimental
General Synthesis
Chemicals were purchased from Sigma-Aldrich, Acros, or Alfa Aesar. Anhydrous solvents were purchased from Sigma-Aldrich. TLC was performed on precoated plates (Merck Aluminum sheets silica 60 F254, art No. 5554). Chromatograms were visualised under UV light and by dipping plates into phosphomolybdic acid in EtOH, followed by heating. Flash column chromatography was performed using RediSep Rf disposable flash columns on an ISCO CombiFlash Rf automated flash chromatography machine. Reverse phase chromatography was performed on LiChroprep RP-18 (25-40 μm, Merck) using a BioLogic LP system (BioRad), eluting at 5 mL/min with a gradient of 0−10% MeCN in 0.05 M triethylammonium bicarbonate (TEAB) buffer, collecting 7 mL fractions. Fractions containing the target polyphosphate were identified using a modification of the Briggs phosphate assay.62 The purity of all of the final compounds used in biological assays were assessed by HPLC and found to be >95% pure (vide infra and HPLC data in Supporting Information). Proton 1H NMR and COSY spectra were recorded on a Bruker Avance III (400 MHz) spectrometer. Proton chemical shifts are reported in ppm (δ) relative to internal tetramethylsilane (TMS, δ 0.0 ppm) or with the solvent reference relative to TMS employed as the internal standard (CDCl3, δ 7.26 ppm; CD3OD, δ 3.31 ppm). The following abbreviations are used to describe the multiplicity of the chemical shifts: br, broad; s, singlet; d, doublet; dd, double doublet; q, quartet; m, multiplet; t, triplet. 13C and HSQC spectra were recorded on a Bruker Avance III (100 MHz) spectrometer with complete proton decoupling. Carbon chemical shifts are reported in ppm (δ) relative to TMS with the respective solvent resonance as the internal standard (CDCl3, δ 77.0 ppm, d4-MeOH, δ 49.0 ppm). 31P NMR spectra were recorded on a Bruker Avance III (162 MHz) spectrometer with complete proton decoupling. Phosphorus chemical shifts are reported in ppm (δ) relative to an 85% H3PO4 external standard (H3PO4, δ 0.0 ppm). All NMR data were collected at 25 °C. Optical rotations were measured at ambient temperature using an Optical Activity Ltd. AA-10 polarimeter in a cell volume of 5 cm3, and specific rotation is given in 10−1 deg cm3 g−1. Melting points were determined using a Stanford Research Systems Optimelt MPA100 automated melting point system and are uncorrected. Mass spectra were recorded on a Thermo Orbitrap Exactive Mass Spectrometer. All reactions were carried out under an argon atmosphere employing oven-dried glassware unless stated otherwise.
Methyl α-l-glucopyranoside (8)
In an modified version of the Li et al.63 procedure, l- glucose (850 mg, 4.7 mmol) was dissolved in anhydrous MeOH (6.5 mL). A solution of hydrogen chloride was prepared by adding acetyl chloride (0.25 mL) to anhydrous MeOH (1.5 mL) at 0°C and this solution was added dropwise to the glucose reaction solution. The reaction was refluxed for 5 days while under nitrogen before the MeOH was evaporated to yield the crude product. The product was recrystallized from EtOH as a white crystalline solid, which contained approximately 5% of the β anomer. The product was recrystallized from EtOH again to yield the pure α anomer of the product as white crystals (414 mg, 2.13 mmol, 45% yield). mp (EtOH) 167.2-168.1°C (Lit.64 mp (EtOH) 161-163 °C); [α]23D - 168.4 (c= 1.00, MeOH) [Lit.64 [α]d -161 (c= 1.0, MeOH)]; 1H NMR (CD3OD, 400 MHz): δ 4.67 (d, J= 3.8 Hz, 1H, H-1), 3.81 (dd, J= 11.8, 2.4 Hz, 1H, H-6), 3.67 (dd, J= 11.8, 5.8 Hz, 1H, H-6), 3.61 (t, J= 9.2 Hz, 1H, H-3), 3.55-3.50 (m, 1H, H-5), 3.41 (s, 3H, OMe), 3.38 (dd, J= 9.7, 3.8 Hz, 1H, H-2), 3.27 (dd, J= 10.0, 9.0 Hz, 1H, H-4); 13C NMR (CD3OD, 100 MHz): δ 101.2 (C-1), 75.1 (C-3), 73.54 (C-5), 73.53 (C-2), 71.8 (C-4), 62.7 (C-6), 55.5 (OMe).
Methyl 3-O-benzyl-4,6-O-benzylidene-α-l-glucopyranoside (12)
Methyl α-l-glucopyranoside (8) (93 mg, 0.480 mmol) was dissolved in dry pyridine (1 mL) and put under argon. To this solution, trimethylsilyl chloride (0.30 mL, 2.39 mmol, 5 equiv) was added dropwise. The solution was allowed to stir for 22 h at room temperature. The reaction was then diluted with EtOAc and washed with water (2 x 30 mL). The organic phase was dried over MgSO4 and concentrated in vacuo to yield the per-silylated glucopyranoside crude product. The per-silyslated product was not purified, but NMR was used to confirm that the reaction had proceeded to completion. The per-silyslated product was dissolved and co-evaporated twice with toluene before being dissolved in dry DCM (0.7 mL) and put under argon. To this solution, benzaldehyde (0.15 mL, 1.42 mmol, 3 equiv) was added and the reaction was cooled in an ice bath. To this chilled solution, iron (III) chloride hexahydrate (3.4 mg, 0.013 mg, 0.026 equiv) dissolved in MeCN (0.12 mL) was added dropwise. Triethylsilane (0.08 mL, 0.528 mmol, 1.1 equiv) was then added dropwise and the reaction was allowed to warm to room temperature and stir for 1.5 h. After this time, the solution was diluted with EtOAc (50 mL), washed with sat. NaHCO3 solution (50 mL), and extracted from the aqueous phase twice more with EtOAc (2 x 30 mL). The combined organic phases were dried over MgSO4 and concentrated in vacuo to yield the crude product. The product was purified with flash chromatography (petroleum ether/EtOAc, 0-100%) to yield the pure product as a white solid (140.7 mg, 0.378 mmol, 79% yield); mp 184.0-185.3 °C; [α]21d -87.5 (c= 1.00, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.51-7.27 (m, 10H, Ar), 5.57 (s, 1H, H-7), 4.97 (d, J = 11.6 Hz, 1H, CH2Ph), 4.82 (d, J= 3.9 Hz, 1H, H-1), 4.79 (d, J = 11.6 Hz, 1H, CH2Ph), 4.30 (dd, J= 4.3, 9.8 Hz, 1H, H-6), 3.87-3.71 (m, 4H, H-2, H-3, H-5, H-6), 3.65 (t, J = 9.2 Hz, 1H, H-4), 3.45 (s, 3H, OMe), 2.29 (d, J= 7.2 Hz, 1H, OH); 13C NMR (CDCl3, 100 MHz): δ 138. 6 (Ar), 137.5 (Ar), 129.1 (Ar), 128.6 (Ar), 128.4 (Ar), 128.2 (Ar), 127.9 (Ar), 126.2 (Ar), 101.4 (C-7), 100.0 (C-1), 82.1 (C-4), 79.0 (C-2 or 3 or 5), 75.0 (CH2Ph), 72.6 (C-2 or 3 or 5), 69.2 (C-6), 62.7 (C-2 or 3 or 5), 55.6 (OMe); HRMS (ESI): m/z calcd [M+Na]+ for C21H24O6: 395.14651, found: 395.14658.
Methyl 3-O-benzyl-α-l-glucopyranoside (13)
Methyl 3-O-benzyl-4,6-O-benzylidene-α- l-glucopyranoside (12) (135.7 mg, 0.364 mmol) was dissolved in MeOH (3 mL). To this solution, water (0.15 mL) and 1 M HCl (aq) (0.3 mL) were added. The reaction was heated to reflux for 3 h. After this time, the reaction was quenched with the addition of NaHCO3 (aq) (25.2 mg in 5 mL water). The solution was concentrated in vacuo and the residue was dissolved and co-evaporated with toluene twice to yield the crude product. The product was purified with flash chromatography (pet ether/EtOAc, 0-100%) to yield the pure product as a white solid (96.3 mg, 0.339 mmol, 93% yield). mp 91.2-93.8 °C; [α]22D -89.5 (c= 0.93, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.39-7.29 (m, 5H, Ar), 5.03 (d, J= 11.5 Hz, 1H, CH2Ph), 4.76 (d, J= 3.9 Hz, 1H, CH2Ph), 4.73 (d, J= 11.5 Hz, 1H, H-1,), 3.88-3.75 (m, 2H, H-6 x2), 3.70-3.52 (m, 4H, H-2, H-3, H-4, H-5), 3.44 (s, 3H, OMe), 2.30 (d, J= 2.4 Hz, 1H, OH), 2.14 (d, J= 9.2 Hz, 1H, OH), 1.93 (dd, J= 5.7, 7.2 Hz, 1H, OH); 13C NMR (CDCl3, 100 MHz): δ 138.6 (Ar), 128.8 (Ar), 128.1 (Ar), 99.7 (C-1), 82.8 (C-3), 75.1 (CH2Ph), 72.9 (C-2), 71.1 (C-5), 70.2 (C-4), 62.5 (C-6), 55.5 (OMe); HRMS (ESI): m/z calcd [M+Na]+ for C14H20O6: 307.11521, found: 307.11515.
Methyl 3-O-benzyl-α-l-glucopyranoside 2,4,6-tris(dibenzyl phosphate) (14)
Methyl 3-O-benzyl-α-l-glucopyranoside (13) (96.3 mg, 0.339 mmol) was dissolved in dry DCM (4 mL) and the solution was put under argon. 5-Phenyl-1H-tetrazole (297 mg, 2.03 mmol, 6 equiv) was added to the solution, followed by dibenzyl diisopropylphosphoramidite (0.55 mL, 1.52 mmol, 4.5 equiv). The reaction was allowed to stir at room temperature overnight. The next day, after the confirmation of successful phosphitylation with 31PNMR, the reaction flask was cooled to -78°C and mCPBA (502 mg, 70% purity, 2.03 mmol, 6 equiv.) was added. The reaction was allowed to stir at room temperature for 10 min before the solution was diluted with EtOAc (50 mL), washed with 10% Na2SO3 solution (2 x 30 mL), dried over MgSO4 and concentrated to yield the crude product. The crude product was purified with flash chromatography (pet ether/EtOAc, 0-100%) to yield the pure product as a colorless oil (144.2 mg, 0.135 mmol, 40% yield). [α]21D -38.8 (c= 1.01, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.34-7.08 (m, 35H, Ar), 5.03 (s, 2H, CH2Ph x2), 5.01 (s, 2H, CH2Ph x2), 4.98 (d, J= 3.2 Hz, 1H, CH2Ph), 4.96 (d, J= 3.2 Hz, 1H, CH2Ph), 4.94-4.85 (m, 6H, H-1, CH2Ph x5), 4.80-4.75 (m, 3H, CH2Ph x3), 4.42-4.18 (m, 4H, H-2, H-3, H-6 x2), 3.98 (t, J= 9.4 Hz, 1H, H-4), 3.86 (dd, J= 10.0, 5.2 Hz, 1H, H-5), 3.28 (s, 3H, OMe); 31P NMR (CDCl3, 162 MHz): δ -0.99, -1.74, -1.86; 13C NMR (CDCl3, 100 MHz): δ 138.1 (Ar), 136.0 (Ar), 135.9 (Ar), 135.8-135.6 (m, Ar), 128.7-128.5 (m, Ar), 128.3 (Ar), 128.0 (m, Ar), 127.9 (Ar), 127.7 (Ar), 127.5 (Ar), 97.6 (C-1), 78.4-78.3 (m, C-4), 76.8-76.7 (m, C-2 or 3), 75.1 (C-2 or 3, CH2Ph), 69.8-69.4 (m, CH2Ph), 69.0-68.9 (m, C-5), 66.1 (d, J= 5.1 Hz, C-6), 55.7 (OMe); HRMS (ESI): m/z calcd [M+H]+ for C56H59O15P3: 1065.31396, found: 1065.31280.
Methyl α-l-glucopyranoside 2,4,6-trisphosphate triethylammonium salt (3)
Methyl 3-O-benzyl-α-l-glucopyranoside 2,4,6-tris(dibenzyl phosphate) (14) (141.9 mg, 0.133 mmol) was dissolved in MeOH (7.1 mL). Ultrapure water (0.71 mL) was added dropwise to the solution, ensuring that the precipitate formed upon addition was able to dissolve back into solution. Pd(OH)2/C (20%, ≥50% wet, 71.0 mg) was added to the solution and the reaction flask was flushed with hydrogen. The reaction was allowed to stir at room temperature for 24 h, after which the catalyst was filtered off and the collected filtrate was evaporated to yield the product as a free acid. No purification steps were deemed to be necessary, but triethylamine was added to sharpen the phosphorus NMR signals and to convert the product from the free acid into the triethylammonium salt. The product was concentrated in vacuo, lyophilised and collected as a colorless glass (96.1 mg, 0.120 mmol, 90% yield). [α]22D -46.5 (c= 0.88, MeOH); 1H NMR (CD3OD, 400 MHz): δ 4.90 (d, J= 3.0 Hz, 1H, H-1), 4.29-4.18 (m, 2H, H-3, H-6), 4.05-3.97 (m, 3H, H-2, H-4, H-6), 3.68 (d, J= 10.0 Hz, 1H, H-5), 3.38 (s, 3H, OMe), 3.14 (q, J= 7.3 Hz, approx. 18H, TEA CH2CH3), 1.30 (t, 7.3 Hz, approx. 27H, TEA CH2CH3); 31P NMR (CD3OD, 162 MHz): δ 1.90, 1.71, 1.28; 13C NMR (CD3OD, 100 MHz): δ 100.2 (d, J= 4.0 Hz, C-1), 76.3 (d, J= 5.1 Hz, C-2 or 4), 74.6 (d, J= 4.0 Hz, C-2 or 4), 73.5 (d, J= 4.9 Hz, C-3), 71.0-71.2 (m, C-5), 63.9 (d, J= 4.7 Hz, C-6), 55.6 (OMe), 46.9 (TEA CH2CH3), 9.4 (TEA CH2CH3). HRMS (ESI): m/z calcd [M-H]- for C7H17O15P3: 432.97075, found: 432.97065.
Methyl 4,6-O-benzylidene-α-l-glucopyranoside (9)
In a version of the Tseberlidis et al.65 method, methyl α-l-glucopyranoside (8) (100 mg, 0.514 mmol) was suspended in dry MeCN (1.7 mL) and put under a nitrogen atmosphere. To this suspension, benzaldehyde dimethyl acetal (0.24 mL, 1.55 mmol, 3 equiv) and catalytic camphor-10-sulphonic acid (1.6 mg, 0.0068 mmol) were added and the reaction was allowed to stir at room temperature overnight. After 24 h, the reaction was neutralised with a few drops of triethylamine and evaporated to yield the crude product as a white crystalline solid. The crude product was purified through flash chromatography (petroleum ether/EtOAc, 0-100%) and the pure product was collected as a white solid (132.6 mg, 0.470 mmol, 91 % yield). mp 163.1-164.4 °C (Lit.64 mp 161-162 °C); [α]21D -110.5 (c= 0.69, CDCl3) [Lit.64 [α]D -95 (c= 1.0, MeOH)]; 1H NMR (CDCl3, 400 MHz): δ 7.51-7.48 (m, 2H, Ar), 7.40-7.34 (m, 3H, Ar), 5.53 (s, 1H, H-7), 4.79 (d, J= 4.0 Hz, 1H, H-1), 4.29 (dd, J= 9.6, 4.3 Hz, 1H, H-6), 3.93 (apt td, J= 9.3, 2.2 Hz, 1H, H-3), 3.84-3.78 (m, 1H, H-5), 3.75 (apt q, J= 10.3 Hz, 1H, H-6), 3.63 (apt td, J= 9.3, 3.9 Hz, 1H, H-2), 3.49 (apt t, J= 9.3 Hz, 1H, H-4), 3.46 (s, 3H, OMe), 2.76 (d, J= 2.2 Hz, 1H, OH), 2.30 (d, J= 9.5 Hz, 1H, OH); 13C NMR (CDCl3, 100 MHz): δ 137.2 (Ar), 129.4 (Ar), 128.5 (Ar), 126.4 (Ar), 102.1 (C-7), 99.9 (C-1), 81.1 (C-4), 73.0 (C-2), 72.0 (C-3), 69.1 (C-6), 62.5 (C-5), 55.7 (OMe).
Methyl 4-O-benzyl-α-l-glucopyranoside (10)
Methyl 4-O-benzyl-α-l-glucopyranoside was made as described for methyl 4-O-benzyl-α-d-glucopyranoside (21) using both method A to generate large amounts of impure product and method B to generate smaller amounts of pure product (26 mg, 37% yield). mp 128.5-132.0 (Lit.66 mp 125-127 °C); [α]25D -142.5 (c= 0.3, MeOH) [Lit.66 [α]25D -144.2 (c= 1.2, MeOH)]. 1H NMR (CDCl3, 400MHz): δ 7.37-7.27 (m, 5H, Ar), 4.87 (AB, J= 11.5 Hz, 1H, CH2Ph), 4.75 (d, J= 3.9 Hz, 1H, H-1), 4.72 (AB, J= 11.4 Hz, 1H, CH2Ph), 3.86 (t, J= 9.2 Hz, 1H, H-3), 3.83 (ABX, J= 11.8, 2.6 Hz, 1H, H-6), 3.75 (ABX, J= 11.8, 3.8 Hz, H-6), 3.67-3.62 (m, 1H, H-5), 3.50 (dd, J= 3.9, 9.4 Hz,1H, H-2), 3.45 (t, J= 9.4 Hz, 1H, H-4), 3.40 (s, 3H, OMe); 13C NMR (CDCl3, 100 MHz): δ 138.3 (Ar), 128.7 (Ar), 128.2 (Ar), 127.9 (Ar), 99.2 (C-1), 77.3 (C-4), 75.3 (C-3), 74.8 (CH2Ph), 72.9 (C-2), 70.9 (C-5), 62.1 (C-6), 55.5 (OMe).
Methyl 4-O-benzyl-α-l-glucopyranoside 2,3,6-tris(dibenzyl phosphate) (11)
Methyl 4-O-benzyl-α-l-glucopyranoside (10) (65.5 mg, 0.230 mmol) was dissolved in dry DCM (2 mL) and put under argon. 5-Phenyl-1H-tetrazole (202 mg, 1.38 mmol, 6 equiv) was added to the solution followed by dibenzyl diisopropylphosphoramidite (0.36 mL, 1.04 mmol, 4.5 equiv). The reaction was allowed to stir at room temperature overnight. The following day, the reaction was cooled to -78°C and mCPBA (70% pure, 342 mg, 1.38 mmol, 6 equiv) was added. The reaction was allowed to stir for 10 min at room temperature before it was diluted with EtOAc (50 mL) and washed twice with 10% Na2SO3 solution (2 x 30 mL). The organic layer was dried over MgSO4 and concentrated to yield the crude product. The residue was purified with flash chromatography (petroleum ether/EtOAc, 0-100%) to yield the pure product as a colorless oil (210.5 mg, 0.198 mmol, 86% yield). [α]21d -42.3 (c= 1.06, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.33-7.09 (m, 35H, Ar), 5.05-4.81 (m, 15H, H-1, H-3, CH2Ph x13), 4.46 (d, J= 10.7 Hz, 1H, CH2Ph), 4.25 (ddd, J=13.3, 6.2, 3.6 Hz, 1H, H-2), 4.19-4.16 (m, 2H, H-6), 3.75 (dq, J= 10.0, 2.3 Hz, 1H, H-5), 3.50 (t, J= 9.5 Hz, 1H, H-4), 3.24 (s, 3H, OMe); 31P NMR (CDCl3, 162 MHz): δ -0.76, -1.32, -2.01; 13C NMR (CDCl3, 100 MHz): δ 137.6 (Ar), 136.1-135.7 (m, Ar), 128.7-127.8 (m, Ar), 97.6 (C-1), 78.4 (dd, J= 8.7, 6.5 Hz, C-3), 76.2 (C-4), 75.3 (dd, J= 4.7, 3.2 Hz, C-2), 74.6 (CH2Ph), 69.8 (d, J= 5.6 Hz, CH2Ph), 69.6-69.4 (m, CH2Ph), 69.1 (d, J= 8.3 Hz, C-5), 65.8 (d, J=5.6 Hz, C-6), 55.5 (OMe); HRMS (ESI): m/z calcd [M+H]+ for C56H59O15P3: 1065.31396, found: 1065.31297.
Methyl α-l-glucopyranoside 2,3,6-trisphosphate triethylammonium salt (2)
Methyl 4-O-benzyl-α-l-glucopyranoside 2,3,6-tris(dibenzyl phosphate) (11) (60 mg, 0.056 mmol) was dissolved in MeOH (3 mL). To this solution, ultrapure water (0.3 mL) was added dropwise, ensuring that the white precipitate that formed returned to solution. Pd(OH)2/C (30 mg, 20% wt) was added to the solution and the flask was flushed with hydrogen. The reaction was left to stir under hydrogen at room temperature overnight. The palladium catalyst was filtered off with a PTFE filter and the solution was concentrated to yield the product as a free acid. No purification was deemed necessary. The free acid was converted to the triethylammonium salt through the addition of triethylamine to the free acid followed by concentration in vacuo. The product was lyophilised and collected as a colourless glass (44.5 mg, 0.056 mmol, 100% yield). [α]21d -37.6 (c= 1.00, MeOH); 1H NMR (CDCl3, 400 MHz): δ 4.92 (d, J= 3.6 Hz, 1H, H-1), 4.35 (q, J= 8.7 Hz, 1H, H-3), 4.17 (ddd, J= 11.1, 5.2, 2.0 Hz, 1H, H-6), 4.07-3.98 (m, 2H, H-2, H-6), 3.73-3.68 (m, 1H, H-5), 3.55 (dd, J= 9.8, 8.7 Hz, 1H, H-4), 3.39 (s, 3H, OMe), 3.09 (q, J= 7.3 Hz, approx. 18H, TEA CH2CH3), 1.27 (t, J= 7.3 Hz, approx. 27H, TEA CH2CH3); 31P NMR (CDCl3, 162 MHz): δ 2.50, 1.53, 1.15; 13C NMR (CDCl3, 100 MHz): δ 100.5 (C-1), 78.5 (C-3), 75.5 (C-2), 72.4 (d, J= 8.7 Hz, C-5), 72.0 (C-4), 65.7 (d, J= 4.9 Hz, C-6), 55.4 (OMe), 47.0 (TEA CH2CH3), 9.4 (TEA CH2CH3). HRMS (ESI): m/z calcd [M-H]- for C7H17O15P3: 432.97075, found: 432.97067.
2,6-Di-O-benzyl-d-glucopyranose (16)
Allyl 2,6-di-O-benzyl-d-glucopyranoside (15) (60 mg, 0.133 mmol) as synthesized in the method outlined by Jenkins et al.30 was dissolved in dry MeOH (1.54 mL). To this solution, PdCl2 (6.2 mg, 0.03 mmol, 0.25 equiv) was added and the reaction was allowed to stir at room temperature for 6 hours with a drying tube affixed to the flask. After this time, the reaction was quenched with the addition of excess NaHCO3 and allowed to stir for 5 minutes before being filtered through celite and concentrated to yield the crude product. The product of this reaction could not be successfully purified, although following phosphorylation (see below), the products could be successfully isolated.
2,6-Di-O-benzyl-α-d-glucopyranosyl 1,3,4-tris(dibenzylphosphate) (17) and 2,6-di-O-benzyl-β-d-glucopyranosyl 1,3,4-tris(dibenzylphosphate) (18)
2,6-di-O-benzyl-d-glucopyranose (16) (154.7 mg, 0.429 mmol) was added to dry DCM (4.5 mL). To this suspension, 5-phenyl-1H-tetrazole (376 mg, 2.58 mmol, 6 equiv) was added, followed by dibenzyl diisopropylphosphoramidite (0.67 mL, 1.93 mmol, 4.5 equiv). The reaction was allowed to stir under argon at room temperature overnight, after which it was cooled to - 78°C and mCPBA (70% purity, 636 mg, 2.58 mmol, 6 equiv) was added. The reaction was then diluted with EtOAc (100 mL) and washed twice with 10% Na2SO3 solution (2 x 30 mL). The organic phase was dried over MgSO4 and concentrated to yield the crude product. The product was purified using flash chromatography (petroleum ether/EtOAc, 0-100%). The stereoisomers of the product were partially isolated and collected as colorless oils (total: 276.6 mg, 0.242 mmol, 56% yield; α-epimer: 70.4 mg, 0.062 mmol, 14% yield; β-epimer: 92.4 mg, 0.081 mmol, 19% yield; remaining unseparated mix of epimers: 113.8 mg, 0.100 mmol, 23% yield).
2,6-Di-O-benzyl-α-d-glucopyranosyl 1,3,4-tris(dibenzylphosphate) (17)
Rf (EtOAc) 0.63; [α]20d 2.68 (c= 1.01, chloroform); 1H NMR (CDCl3, 400 MHz): δ 7.35-7.09 (m, 40H, Ar), 5.91 (dd, J= 3.3, 7.0 Hz, 1H, H-1α), 5.07-4.81 (m, 13H, H-3, CH2Ph x12), 4.72-4.61 (m, 3H, H-4, CH2Ph x2), 4.44 (d, J= 12.0 Hz, 1H, CH2Ph), 4.31 (d, J= 12.0 Hz, 1H, CH2Ph), 3.96 (dq, J= 10.0, 1.6 Hz, 1H, H-5), 3.70 (dd, J= 11.2, 3.9 Hz, 1H, H-6), 3.65 (dt, J= 9.7, 3.1 Hz, 1H, H-2), 3.54 (dd, J= 11.2, 1.8 Hz, 1H, H-6); 31P NMR (CDCl3, 162 MHz): δ -1.66, -2.31, -2.50; 13C NMR (CDCl3, 100 MHz): δ 138.0 (Ar), 137.0 (Ar), 136.2-135.6 (m, Ar), 128.6-127.6 (m, Ar), 94.8 (d, J= 5.6 Hz, C-1), 77.9-77.8 (m, C-3), 77.0 (m, C-2), 73.7-73.6 (m, C-4), 73.3 (CH2Ph), 73.0 (CH2Ph), 71.6 (C-5), 70.0-69.9 (m, CH2Ph), 69.7-69.6 (m, CH2Ph), 69.5-69.4 (m, CH2Ph), 67.5 (C-6); HRMS (ESI): m/z calcd [M+Na]+ for C62H63O15P3: 1163.32720, found: 1163.32495.
2,6-Di-O-benzyl-β-d-glucopyranosyl 1,3,4-tris(dibenzylphosphate) (18)
Rf (EtOAc) 0.57; [α]21D 35.7 (c= 0.71, chloroform); 1H NMR (CDCl3, 400 MHz): δ 7.31-7.03 (m, 40H, Ar), 5.31 (t, J= 7.2 Hz, 1H, H-1β), 5.04-4.87 (m, 11H, CH2Ph x11), 4.81-4.60 (m, 5H, C-3, C-4, CH2Ph x3), 4.42 (d, J= 12.0 Hz, 1H, CH2Ph), 4.31 (d, J= 12.0 Hz, 1H, CH2Ph), 3.77-3.55 (m, 4H, C-2, C-5, C-6 x2); 31P NMR (CDCl3, 162 MHz): δ -1.90, -2.22, -2.59; 13C NMR (CDCl3, 100 MHz): δ 138.0 (Ar), 137.7 (Ar), 136.1-135.4 (m, Ar), 128.6-127.6 (m, Ar), 98.6 (C-1), 80.1 (m, C-3 or 4), 79.7-79.6 (m, C-2 or 5), 74.5 (d, J= 3.5 Hz, C-3 or 4), 74.1 (CH2Ph), 74.0-73.9 (m, C-2 or 5), 73.3 (CH2Ph), 70.0 (d, J= 5.8 Hz, CH2Ph), 69.8-69.7 (m, CH2Ph), 69.6-69.5 (m, CH2Ph), 68.1 (C-6); HRMS (ESI): m/z calcd [M+Na]+ for C62H63O15P3: 1163.32720, found: 1163.32505.
α-d-Glucopyranosyl 1,3,4-trisphosphate triethylammonium salt (6)
α-d-glucopyranosyl 1,3,4-trisphosphate was prepared with 2,6-di-O-benzyl-α-d-glucopyranosyl 1,3,4-tris(dibenzylphosphate) (17) (31.9 mg, 0.028 mmol) using the same hydrogenation method as described for the synthesis of β-d-glucopyranosyl 1,3,4-trisphosphate (6). The crude sodium salt of the product was purified by ion pair column chromatography on RP18 and lyophilised to yield the triethylamine salt of the product as a colorless glass (8.0 mg, 0.011 mmol, 39% yield). [α]20D 33.9 (c=0.97, methanol); 1H NMR (CD3OD, 400 MHz): δ 5.58 (dd, J= 7.0, 3.6 Hz, 1H, H-1), 4.45 (q, J= 9.0 Hz, 1H, H-3), 4.12 (q, J= 9.8 Hz, 1H, H-4), 3.98-3.91 (m, 2H, H-5, H-6), 3.76-3.72 (m, 1H, H-6), 3.61 (ddd, J= 9.5, 3.5, 2.4 Hz, 1H, H-2), 3.16 (q, J= 7.3 Hz, approx. 18H, TEA CH2CH3), 1.31 (t, J= 7.3 Hz, approx. 27H, TEA CH2CH3); 31P NMR (CD3OD, 162 MHz): δ 2.06, 2.06, -0.62; 13C NMR (CD3OD, 100 MHz): δ 96.3 (d, J= 5.4 Hz, C-1), 79.2-79.1 (m, C-3), 73.8-73.6 (m, C-2, C-4, C-5), 62.1 (C-6), 47.3 (TEA CH2CH3), 9.2 (TEA CH2CH3). HRMS (ESI): m/z calcd [M-H]- for C6H15O15P3: 418.9551, found: 418.95422.
β-d-Glucopyranosyl 1,3,4-trisphosphate triethylammonium salt (7)
2,6-di-O-benzyl-β-d-glucopyranosyl 1,3,4-tris(dibenzylphosphate) (18) (23.3 mg, 0.020 mmol) was dissolved in MeOH (1.5 mL). To this solution, ultrapure water (0.15 mL) was added dropwise, ensuring that the precipitate that formed upon addition returned to solution. NaHCO3 (5.15 mg, 0.061 mmol, 3 equiv) was then added, followed by 20% Pd(OH)2/C (≥50% wet, 11.7 mg). The reaction flask was flushed with hydrogen and left to stir at room temperature for 24 h. The catalyst was then filtered off and the collected filtrate was evaporated to yield the crude product as a sodium salt. The product was purified by ion pair column chromatography on RP18 and lyophilised to yield the triethylamine salt of the product as a colorless glass (6.7 mg, 0.008 mmol, 40% yield). [α]21D 3.76 (c=0.61, methanol); 1H NMR (CD3OD, 500 MHz): δ 4.98 (t, J= 7.6 Hz, 1H, H-1), 4.23 (q, J= 8.8 Hz, 1H, H-3), 4.08 (q, J= 9.8 Hz, 1H, H-4), 3.89 (dd, J= 12.7, 4.4 Hz, 1H, H-6), 3.84 (dd, J= 12.7, 2.1 Hz, 1H, H-6), 3.43-3.38 (m, 2H, H-2, H-5), 3.13 (q, J= 7.0 Hz, approx. 18H, TEA CH2CH3), 1.29 (t, J= 7.2 Hz, approx. 27H, TEA CH2CH3); 31P NMR (CD3OD, 162 MHz): δ 1.38, 1.06, - 0.77; 13C NMR (CD3OD, 125 MHz): δ 99.3 (C-1), 81.4 (C-3), 77.9 (C-2 or 5), 76.2 (C-2 or 5), 73.8 (C-4), 62.4 (C-6), 47.2 (TEA CH2CH3), 9.3 (TEA CH2CH3). HRMS (ESI): m/z calcd [M-H]- for C6H15O15P3: 418.9551, found: 418.95425.
Methyl 4-O-benzyl-α-d-glucopyranoside (21)
Method A: In a version of the Daragics et al.67 method, methyl 4,6-O-benzylidene-α-d-glucopyranoside (100 mg, 0.354 mmol) was dissolved in dry DCM (5.3 mL) and put under argon. The solution was cooled in an ice bath and borane-THF (1 M, 1.8 mL, 1.77 mmol, 5 equiv) was added, followed by a solution of AlCl3 (94.4 mg, 0.708 mmol, 2 equiv) in dry diethyl ether (0.9 mL). The solution was allowed to gradually warm to room temperature and then stir for 24 h. After this time, the reaction was quenched with the addition of triethylamine (0.2 mL) followed by MeOH (0.9 mL). The reaction solution was concentrated in vacuo to form a solid residue. This residue was dissolved in DCM (50 mL) and washed with 1 M HCl(aq), sat. NaHCO3(aq) and water. The combined aqueous washes were also extracted three times with EtOAc and the organic layers were combined, dried over MgSO4 and concentrated to yield the crude product. The crude product was purified through silica column chromatography using petroleum ether and EtOAc. It should be noted that this product was not entirely pure as a very small amount of methyl 6-O-benzyl-α-d-glucopyranoside was generated as well. This regioisomer could not be separated from the desired product (although separation of the regioisomers post-phosphorylation was achievable).
Method B: Using a version of the Shie et al.37 procedure, methyl 4,6-O-benzylidene α-d-glucopyranoside (100 mg, 0.355 mmol) was added to borane-THF (1M, 1.8 mL, 1.8 mmol, 5 equiv) and the reaction was put under argon. The solution was allowed to stir for 10 min before lanthanum triflate (31.2 mg, 0.053 mmol, 0.15 equiv) was added and the reaction was allowed to stir at room temperature for a week. The reaction was then cooled to 0°C and the reaction was quenched with triethylamine (0.5 mL, 1 equiv), followed by MeOH (0.7 mL). The reaction was concentrated in vacuo and co-evaporated with MeOH twice before the crude product was isolated as a white solid. The product was purified with flash chromatography (1. petroleum ether/EtOAc, 0-100% and 2. DCM/EtOAc, 0-100%). As impurities were still present, an aqueous work up was carried out. The product was dissolved in EtOAc and washed with 1 M HCl(aq), sat. NaHCO3 and water. The aqueous washes were extracted with EtOAc again and the organic phases were combined, dried over MgSO4 and concentrated to yield the pure product as a white solid (30.8 mg, 0.108 mmol, 31% yield). mp 123.9-129.0 °C (Lit.68 mp 126-127 °C); [α]21D 116.2 (c=1.47, CHCl3) [Lit.68 [α]D 154.1 (c= 1, CHCl3)]; 1H NMR (CDCl3, 400MHz): δ 7.36-7.28 (m, 5H, Ar), 4.86 (AB, J= 11.4 Hz, 1H, CH2Ph), 4.75 (d, J= 3.9 Hz, 1H, H-1), 4.72 (AB, J= 11.4 Hz, 1H, CH2Ph), 3.86 (t, J= 9.2 Hz, 1H, H-3), 3.83 (ABX, J= 11.6, 2.6 Hz, 1H, H-6), 3.75 (ABX, J= 11.9, 3.6 Hz, H-6), 3.63 (apt dt, J= 9.8, 3.3 Hz, 1H, H-5), 3.51 (brs, 1H, H-2), 3.45 (t, J= 9.4 Hz, 1H, H-4), 3.39 (s, 3H, OMe); 13C NMR (CDCl3, 100 MHz): δ 138.3 (Ar), 128.7 (Ar), 128.2 (Ar), 128.1 (Ar), 99.2 (C-1), 77.2 (C-4), 75.1 (C-3), 74.8 (CH2Ph), 72.8 (C-2), 70.9 (C-5), 62.0 (C-6), 55.5 (OMe).
Methyl 4-O-benzyl-α-d-glucopyranoside 2,3,6-tris(dibenzyl phosphate) (22)
Methyl 4-O-benzyl-α-d-glucopyranoside (21) (50 mg, 0.176 mmol) was dissolved in dry DCM (2 mL) and the solution was put under argon. 5-Phenyl-1H-tetrazole (154 mg, 1.06 mmol, 6 equiv) was added to the solution followed by dibenzyl diisopropylphosphoramidite (0.27 mL, 0.792 mmol, 4.5 equiv). The reaction was allowed to stir at room temperature overnight. The following day, the reaction was cooled to -78°C and mCPBA (70% pure, 261 mg, 1.06 mmol, 6 equiv) was added. The reaction was allowed to stir for 10 min at room temperature before it was diluted with EtOAc (50 mL) and washed twice with 10% Na2SO3 solution (2 x 30 mL). The organic layer was dried over MgSO4 and concentrated to yield the crude product. The product was purified with flash chromatography (1. petroleum ether/EtOAc, 0-100% and 2. DCM/EtOAc, 0-100%). The pure product was collected as a colourless oil (81.9 mg, 0.077 mmol, 44% yield). [α]22D 35.7 (c= 1.00, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.33-7.09 (m, 35H, Ar), 5.05-4.82 (m, 15H, H-1, H-3, CH2Ph x13), 4.47 (d, J= 10.7 Hz, 1H, CH2Ph) 4.26 (ddd, J= 9.6, 6.2, 3.6 Hz, 1H, H-2), 4.19-4.16 (m, 2H, H-6 x2), 3.76 (dq, J= 9.6, 2.7 Hz, 1H, H-5), 3.50 (t, J= 9.4 Hz, 1H, H-4), 3.25 (s, 3H, OMe); 31P NMR (CDCl3, 162 MHz): δ -0.76, -1.32, -2.01; 13C NMR (CDCl3, 100 MHz): δ 137.6 (Ar), 136.1-135.7 (m, Ar), 128.7-128.0 (m, Ar), 97.6 (C-1), 78.5-78.4 (m, C-3), 76.2 (C-4), 75.4-75.3 (m, C-5), 74.6 (CH2Ph), 69.8 (d, J= 5.6 Hz, CH2Ph), 69.6-69.4 (m, CH2Ph), 69.1 (d, J= 8.1 Hz, C-5), 65.8 (d, J= 5.5 Hz, C-6), 55.5 (OMe); HRMS (ESI): m/z calcd [M+H]+ for C56H59O15P3: 1065.31396, found: 1065.31364.
Methyl α-d-glucopyranoside 2,3,6-trisphosphate triethylammonium salt (4)
Methyl 4-O-benzyl-α-d-glucopyranoside 2,3,6-tris(dibenzyl phosphate) (22) (55.5 mg, 0.052 mmol) was dissolved in MeOH (2.8 mL). To this solution, ultrapure water (0.28 mL) was added dropwise, ensuring that the white precipitate that formed returned to solution. 20% Pd(OH)2/C (≥50% wet, 27.8 mg) was added to the solution and the flask was flushed with hydrogen. The reaction was left to stir under hydrogen at room temperature overnight. The palladium catalyst was filtered off with a PTFE filter and the solution was concentrated to yield the product as a free acid. No purification was deemed necessary. The free acid was converted to the triethylammonium salt through the addition of triethylamine to the free acid followed by concentration in vacuo. The product was lyophilised and collected as a colourless glass (24.8 mg, 0.034 mmol, 65% yield). [α]22D 38.2 (c= 1.00, MeOH); 1H NMR (CD3OD, 400 MHz): δ 4.91 (d, J= 3.6 Hz, 1H, H-1), 4.35 (q, J= 8.7 Hz, 1H, H-3), 4.17 (ddd, J= 11.0, 5.2, 2.0 Hz, 1H, H-6), 4.07-3.98 (m, 2H, H-2, H-6), 3.72-3.68 (m, 1H, H-5), 3.54 (dd, J= 9.8, 8.8 Hz, 1H, H-4), 3.39 (s, 3H, OMe), 3.11 (q, J= 7.3 Hz, approx. 18H, TEA CH2CH3), 1.28 (t, J= 7.3 Hz, approx. 27H, TEA CH2CH3); 31P NMR (CD3OD, 162 MHz): δ 2.21, 1.25, 0.94; 13C NMR (CD3OD, 100 MHz): δ 100.2 (C-1), 78.5 (t, J= 5.7 Hz, C-3), 75.3 (t, J= 4.9 Hz, C-2), 72.2 (d, J= 8.3 Hz, C-5), 71.7 (C-4), 65.6 (d, J= 5.2 Hz, C-6), 55.5 (OMe), 47.2 (TEA, CH2CH3), 9.1 (TEA, CH2CH3). HRMS (ESI): m/z calcd [M-H]- for C7H17O15P3: 432.97075, found: 432.97076.
Methyl 4,6-O-benzylidene-3-O-benzyl-α-d-glucopyranoside (23)
Synthesised as described in Bourdreux et al.38 using methyl α-d-glucopyranoside (100 mg, 0.515 mmol). The crude product was purified with flash chromatography (petroleum ether/EtOAc, 0-100%) and the pure product was collected as a white solid (143 mg, 0.384 mmol, 74% yield). mp 178.4-181.0 °C (Lit.69 mp 184 °C); [α]22D 87.7 (c= 0.70, CHCl3) [Lit.69 [α]20D 77 (c= 0.91, CHCl3)]; 1H NMR (CDCl3, 400 MHz): δ 7.52-7.27 (m, 10H, Ar), 5.57 (s, 1H, H-7), 4.97 (d, J= 11.6 Hz, 1H, CH2Ph), 4.82 (d, J= 3.8 Hz, 1H, H-1), 4.79 (d, J= 11.6 Hz, 1H, CH2Ph), 4.30 (dd, J= 4.3, 9.8 Hz, 1H, H-6), 3.87-3.71 (m, 4H, H-2, H-3, H-5, H-6), 3.65 (t, 1H, J= 9.1 Hz, H-4), 3.45 (s, 3H, OMe), 2.29 (d, 1H, J= 7.4 Hz, OH); 13C NMR (CDCl3, 100 MHz): δ 138.6 (Ar), 137.4 (Ar), 129.1 (Ar), 128.5 (Ar), 128.4 (Ar), 128.1 (Ar), 127.9 (Ar), 126.1 (Ar), 101.4 (H-7), 100.0 (C-1), 82.1 (C-4), 79.0 (C-2 or 3 or 5), 74.9 (CH2Ph), 72.5 (C-2 or 3 or 5), 69.1 (C-6), 62.7 (C-2 or 3 or 5), 55.5 (OMe).
Methyl 3-O-benzyl-α-d-glucopyranoside (24)
As described in Boettcher et al.70 using methyl 4,6-O-benzylidene-3-O-benzyl-α-d-glucopyranoside (23) (120 mg, 0.322 mmol). The product was purified with flash chromatography (petroleum ether/EtOAc, 0-100%) to yield the pure product as a white solid (75.7 mg, 0.266 mmol, 83% yield). mp 92.1-93.9 °C (Lit.71 mp 85-86 °C); [α]23D 89.9 (c= 1.05, CHCl3) [Lit.71 [α]23D 95.1 (c=1.16, CHCl3)]; 1H NMR (CDCl3, 400 MHz): δ 7.39-7.27 (m, 5H, Ar), 4.99 (AB, J= 11.6, 1H, CH2Ph), 4.74 (AB, J= 11.6 Hz, 1H, CH2Ph), 4.73 (d, J= 3.7 Hz, 1H, H-1), 3.82-3.74 (m, 2H, H-6 x2), 3.67-3.52 (m, 4H, H-2, H-3, H-4, H-5), 3.42 (s, 3H, OMe), 2.74 (d, J= 2.5 Hz, 1H, OH), 2.32 (d, J= 8.7 Hz, 1H, OH), 2.27 (t, J = 6.4 Hz, 1H, OH); 13C NMR (CDCl3, 100 MHz): δ 138.6 (Ar), 128.7 (Ar), 128.10 (Ar), 128.07 (Ar), 99.7 (C-1), 82.8 (C-3), 75.1 (CH2Ph), 72.9 (C-2), 71.2 (C-5), 70.1 (C-4), 62.3 (C-6), 55.5 (OMe).
Methyl 3-O-benzyl-α-d-glucopyranoside 2,4,6-tris(dibenzyl phosphate) (25)
Methyl 3-O-benzyl-α-d-glucopyranoside (24) (51.9 mg, 0.183 mmol) was dissolved in dry DCM (2 mL) and the solution was put under argon. 5-Phenyl-1H-tetrazole (160 mg, 1.10 mmol, 6 equiv) was added to the solution, followed by dibenzyl diisopropylphosphoramidite (0.30 mL, 0.82 mmol, 4.5 equiv). The reaction was allowed to stir at room temperature overnight. The next day, after the confirmation of successful phosphitylation with 31PNMR, the reaction flask was cooled to -78°C and mCPBA (270.7 mg, 70% purity, 1.10 mmol, 6 equiv.) was added. The reaction was allowed to stir at room temperature for 10 min before the solution was diluted with EtOAc (50 mL), washed with 10% Na2SO3 solution (2 x 30 mL), dried over MgSO4 and concentrated to yield the crude product. The product was purified with flash chromatography (petroleum ether/EtOAc, 0-100%) to yield the pure product as a colourless oil (138.1 mg, 0.130 mmol, 71% yield). [α]21D 41.4 (c= 0.61, CHCl3); 1H NMR (CDCl3, 400 MHz): δ 7.35-7.08 (m, 35H, Ar), 5.03 (s, 2H, CH2Ph x2), 5.01 (s, 2H, CH2Ph x2), 4.98 (d, J= 3.2 Hz, 1H, CH2Ph), 4.97 (d, J= 3.2 Hz, 1H, CH2Ph), 4.94-4.85 (m, 6H, H-1, CH2Ph x5), 4.81-4.75 (m, 3H, CH2Ph x3), 4.43-4.18 (m, 4H, H-2, H-3, H-6 x2), 3.98 (t, J= 9.3 Hz, 1H, H-4), 3.86 (dd, J= 10.0, 5.2 Hz, 1H, H-5), 3.28 (s, 3H, OMe); 31PNMR (CDCl3, 162 MHz): δ -0.98, -1.73, -1.85; 13C NMR (CDCl3, 100 MHz): δ 138.1 (Ar), 136.0 (Ar), 135.9 (Ar), 135.8-132.6 (m, Ar), 128.7-128.5 (m, Ar), 128.3 (Ar), 128.0 (m, Ar), 127.8 (Ar), 127.7 (Ar), 127.5 (Ar), 97.6 (C-1), 78.3 (m, C-4), 76.7 (m, C-2 or 3), 75.0 (C-2 or 3, CH2Ph), 69.6 (m, CH2Ph), 69.4 (m, CH2Ph), 68.9 (m, C-5), 66.1 (m, C-6), 55.7 (OMe); HRMS (ESI): m/z calcd [M+H]+ for C56H59O15P3: 1065.31396, found: 1065.31336.
Methyl α-d-glucopyranoside 2,4,6-trisphosphate triethylammonium salt (5)
Methyl 3-O-benzyl-α-d-glucopyranoside 2,4,6-tris(dibenzyl phosphate) (25) (132.4 mg, 0.124 mmol) was dissolved in MeOH (6.6 mL). Ultrapure water (0.66 mL) was added dropwise to the solution, ensuring that the precipitate formed upon addition was able to dissolve back into solution. 20% Pd(OH)2/C (≥50% wet, 66.2 mg) was added to the solution and the reaction flask was flushed with hydrogen. The reaction was allowed to stir at room temperature for 24 h, after which the catalyst was filtered off and the collected filtrate was evaporated to yield the product as a free acid. No purification steps were deemed to be necessary, but triethylamine was added to sharpen the phosphorus NMR signals and to convert the product from the free acid into the triethylammonium salt. The product was concentrated in vacuo before being lyophilised and collected as a colourless glass (86 mg, 0.10 mmol, 94% yield). [α]22D 39.3 (c= 0.43, MeOH); 1H NMR (CD3OD, 400 MHz): δ 4.91 (d, J= 2.8 Hz, 1H, H-1), 4.26-4.16 (m, 2H, H-3, H-6), 4.05-3.98 (m, 3H, H-2, H-4, H-6), 3.70 (brd, J= 9.8 Hz, 1H, H-5), 3.39 (s, 3H, OMe), 3.12 (q, J= 7.5 Hz, approx. 18H, TEA CH2CH3), 1.29 (t, J= 7.5 Hz, approx. 27H, TEA CH2CH3); 31PNMR (CD3OD, 162 MHz): δ 1.99, 1.75, 1.32; 13C NMR (CD3OD, 100 MHz): δ 100.2 (C-1), 76.3 (d, 5.6 Hz, C-2 or 4), 74.6 (d, 3.4 Hz, C-2 or 4), 73.5 (d, 5.5 Hz, C-3), 71.1 (m, C-5), 63.9 (C-6), 55.6 (OMe), 47.0 (TEA CH2CH3), (TEA, CH2CH3) 9.3. HRMS (ESI): m/z calcd [M-H]- for C7H17O15P3: 432.97075, found: 432.97063.
HPLC
For analysis and stability experiments, the sugar phosphates were resolved by anion exchange HPLC on a 3x250 mm CarboPac PA200 column (Dionex) fitted with 3x50 mm guard cartridge of the same material. Compounds were eluted with a gradient derived from buffer reservoirs containing water (A) and 0.6 M methanesulfonic acid (B) delivered at a flow rate of 0.4 mL min-1 according to the following schedule: time (min), % B; 0, 0; 20, 80; 21, 0; 31, 0. Compounds were detected with the phosphate detection reagent of Phillippy and Bland, 1988.72 For this purpose, the column eluate was mixed in a mixing Tee with a solution of 0.1% (w/v) ferric nitrate nonahydrate in 2% (w/v) perchloric acid delivered at a flow rate of 0.2 mL min-1 and passed through a 0.192 mL internal volume knitted reaction coil before transfer to a UV detector set at 290 nm. Typically, samples of 40 μL of 500 μM solutions in water were injected. Data were exported from the Chrom-Nav2 software as x,y data files and redrawn in GraFit.v7.73
Biology methods
Materials
HEK-293 cells with all three Ins(1,4,5)P3R subtypes disrupted using CRISPR/Cas9 technology (HEK-3KO)74 were from Kerafast (Boston, USA). Dulbecco's modified Eagle’s medium/nutrient mixture F-12 with GlutaMAX (DMEM/F-12 Gluta-MAX) and Mag-fluo-4 AM were from ThermoFisher. TransIT-LT1 transfection reagent was from Geneflow (Elmhurst, Lichfield, UK). Most chemicals and foetal bovine serum (FBS) were from Sigma-Aldrich (Gillingham, UK). Cyclopiazonic acid (CPA) was from Tocris (Bristol, UK). G418 was from Formedium (Norfolk, UK). Protease inhibitors cocktail tablets were from Roche. Half-area 96-well black-walled plates were from Greiner. Ins(1,4,5)P3 was from Enzo (Exeter, UK). [3H]-Ins(1,4,5)P3 was from Perkin Elmer.
Cell Culture and Transfection
HEK cells were cultured in DMEM/F-12 GlutaMAX medium supplemented with 10% FBS (37°C in 95% air and 5% CO2). Cells were either passaged or used for experiments when they reached confluence. HEK cells expressing only Ins(1,4,5)P3R1 (HEK-Ins(1,4,5)P3R1) were generated by transfecting HEK-3KO cells with the gene encoding rat Ins(1,4,5)P3R1 (lacking the S1 splice site)27 cloned into pcDNA3.1(-)/Myc-His B plasmid75 using TransIT-LT1 reagent following the manufacturer’s instructions. To generate stable cell lines, cells were passaged 48 h after transfection in medium with G418 (1 mg/mL). Selection was maintained for 2 weeks, and medium was changed every 3 days. Monoclonal cell lines were selected by plating cells (~1 cell/well) into 96-well plates in medium containing G418 (1 mg/mL). After 4 days, wells with only one cell were identified and the cells were allowed to reach confluence. These cell lines were then expanded and their expression of Ins(1,4,5)P3R1 was confirmed by western blot using an anti-Ins(1,4,5)P3R1 antibody.27
Ca2+ Release Assays
The free [Ca2+] within the lumen of the endoplasmic reticulum (ER) was measured using the low-affinity Ca2+ indicator Mag-fluo-4.76,77 The ER of HEK-Ins(1,4,5)P3R1 cells was loaded with the Ca2+ indicator by incubating cells (2.4 x 107 cells/mL, 1 h, 22 °C) in HEPES-buffered saline (HBS; 135 mM NaCl, 5.9 mM KCl, 11.6 mM HEPES, 1.5 mM CaCl2, 11.5 mM glucose, 1.2 mM MgCl2, pH 7.3) supplemented with BSA (1 mg/mL), Pluronic F127 (0.4 mg/mL) and Mag-fluo-4 AM (20 μM). Cells were then suspended in Ca2+-free cytosol-like medium (CLM: 20 mM NaCl, 140 mM KCl, 1 mM EGTA, 20 mM Pipes, 2 mM MgCl2, pH 7.0) and permeabilized with saponin (10 μg/mL, 3 min, 37 °C). Permeabilized cells were centrifuged (650 xg, 3 min), and incubated in CLM (7 min, 37 °C) to allow the Ca2+ stores to empty. Cells were then centrifuged (650 xg, 3 min) and re-suspended in CLM without Mg2+, but supplemented with 375 μM CaCl2 to give a final free [Ca2+] of 220 nM after addition of 1.5 mM MgATP. Cells (~4 x 105/well) were added to poly-l-lysine-coated half-area 96-well black-walled plates. Fluorescence was recorded at 20 °C at intervals of 1.44 s using a FlexStation III plate-reader (Molecular Devices, Sunnyvale, CA, USA) with excitation and emission wavelengths of 485 nm and 520 nm, respectively. MgATP (1.5 mM) was added to initiate Ca2+ uptake, and when the ER had loaded to steady-state (~2.5 min), cyclopiazonic acid (CPA, 10 μM) was added to inhibit the ER Ca2+ pump. Ins(1,4,5)P3 or other ligands were added after a further 60 s. The amount of Ca2+ released was calculated as a percentage of the fluorescence signal from fully loaded stores (Ffull) minus the signal from non-loaded stores (Ffull –Fempty). Results are presented as mean ± SEM from 5-11 independent experiments, each run in duplicate.
[3H]-Ins(1,4,5)P3 binding to cerebellar membranes
Cerebellar membranes, which are rich in Ins(1,4,5)P3R1, were prepared from the cerebella of adult Wistar rats. Frozen cerebella were homogenized at 4° C in homogenization medium (HM: 1 mM EDTA, 50 mM Tris, protease inhibitors, pH 8.3) supplemented with 100 mM NaCl. After centrifugation (130,000 xg, 1 hr, 4° C), the membranes were resuspended in HM (~6 mg protein/mL) and stored at -80° C. Equilibrium-competition binding assays were performed at 4° C in a final volume of 500 μL of Tris-EDTA medium (TEM: 50 mM Tris, 1 mM EDTA, pH 8.3) with [3H]-Ins(1,4,5)P3 (19.3 Ci/mmol, 1.5 nM), competing ligands and 25 μL of membranes.27 After 5 min, during which equilibrium was attained, bound and free ligand were separated by centrifugation (20,000 xg, 5 min, 4° C), the pellet was then rinsed and resuspended in TEM (200 μL) before liquid scintillation counting (1 mL, Ecoscint-A). Non-specific binding, determined by addition of 10 μM Ins(1,4,5)P3 was always <10% of total binding, and <10% of the added 3H-IP3 was bound. Results are presented as mean ± SEM from 3 independent experiments without replicates.
Data analysis
Equilibrium binding results and concentration-effect relationships were fitted to Hill equations (GraphPad Prism, version 5) from which -logIC50 (pIC50) and - logEC50 (pEC50) values were obtained. For equilibrium competition binding assays pKd values were calculated using the Cheng and Prussof equation.78 Because pEC50 and pKd values are normally distributed, these results are presented as means ± SEM from n independent experiments. For comparisons of the ratios between mean values (EC50/Kd), statistical analyses compared the differences between their log values (pEC50 and pKd),79 with the SEM calculated as follows, assuming that the population variances are the same (confirmed using an F test)80:
where, sp is the estimate of the population variance:
where, s1 and s2 are the sample standard deviations, and n1 and n2 are the sample sizes. Although all analyses were performed using log values, for greater clarity we present ratios as the antilogs of the means and the 95% confidence interval.
Statistical analysis used ANOVA followed by Bonferroni's Multiple Comparison Test (GraphPad Prism, version 5). P < 0.05 was considered significant.
EC39.4% release/Kd was calculated because some ligands did not fully release the Ins(1,4,5)P3-sensitive stores. The ratio was calculated using the concentration of each ligand that caused release of 39.4% of the total content of the stores (which is the % released by Ins(1,4,5)P3 at its EC50).
Supplementary Material
Overlays, molecular docking and ligand interaction diagrams for 2, 3, 6, 7; HPLC data for compounds 2-7; stability studies of compounds 6 and 7 (SI-1); and NMR spectral data for all compounds (SI-2). Molecular formula strings (csv). This material is available free of charge via the Internet at http://pubs.acs.org.
Scheme 2.
Synthesis of ligands with phosphate groups at the anomeric carbon: α-d-glucopyranosyl 1,3,4-trisphosphate (6) and β-d-glucopyranosyl 1,3,4-trisphosphate (7)a
aReagents and conditions: (a) MeOH, cat. PdCl2, 6h (b) (1) DCM, 5-phenyl-1H-tetrazole, (BnO)2PN(iPr)2, 20h (2) mCPBA, -78°C - room temperature (c) MeOH:H2O (10:1 v/v), cat. Pd(OH)2/C, H2, NaHCO3, 24h.
Acknowledgments
BVLP (grant 101010) and CWT (grant 101844) are Wellcome Trust Senior Investigators.
Abbreviations
- AdA
Adenophostin A
- ATP
adenosine triphosphate
- COSY
correlation spectroscopy
- mCPBA
meta-chloroperoxybenzoic acid
- CPA
cyclopiazonic acid
- cryo-EM
cryogenic electron microscopy
- CSA
camphorsulfonic acid
- DCM
dichloromethane
- EDTA
ethylenediaminetetraacetic acid
- ER
endoplasmic renticulum
- FBS
fetal bovine serum
- HBS
HEPES buffered saline
- HEK
human embryonic kidney
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HPLC
high performance liquid chromatography
- HRMS
high resolution mass spectrometry
- HSQC
heteronuclear single quantum coherence
- IBC
Ins(1,4,5)P3 binding core
- Ins(1,4,5)P3
d-myo-inositol 1,4,5-trisphosphate
- Ins(1,4,5)P3R
d-myo-inositol 1,4,5-trisphosphate receptor
- NMR
nuclear magnetic resonance
- SAR
structure-activity relationship
- THF
tetrahydrofuran
- TMS
tetramethylsilane
References
- (1).Rossi AM, Taylor CW. IP 3 Receptors – Lessons from Analyses Ex Cellula. J Cell Sci. 2019;132(4) doi: 10.1242/jcs.222463. jcs222463. [DOI] [PubMed] [Google Scholar]
- (2).Foskett JK, White C, Cheung K-H, Mak D-OD. Inositol Trisphosphate Receptor Ca 2+ Release Channels. Physiol Rev. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Berridge MJ. Inositol Trisphosphate and Calcium Signalling Mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- (4).Bosanac I, Alattia J-R, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, et al. Structure of the Inositol 1, 4, 5-Trisphosphate Receptor Binding Core in Complex with Its Ligand. Nature. 2002 Dec;420:3–7. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- (5).Sureshan KM, Riley AM, Thomas MP, Tovey SC, Taylor CW, Potter BVL. Contribution of Phosphates and Adenine to the Potency of Adenophostins at the IP3 Receptor: Synthesis of All Possible Bisphosphates of Adenophostin A. J Med Chem. 2012;55(4):1706–1720. doi: 10.1021/jm201571p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Seo M-D, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, Taylor CW. Structural and Functional Conservation of Key Domains in InsP 3 and Ryanodine Receptors. Nature. 2012;483:108–114. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Paknejad N, Hite RK. Structural Basis for the Regulation of Inositol Trisphosphate Receptors by Ca2+ and IP3. Nat Struct Mol Biol. 2018;25:660–668. doi: 10.1038/s41594-018-0089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Potter BVL, Lampe D. Chemistry of Inositol Lipid Mediated Cellular Signaling. Angew Chemie Int Ed Eng. 1995;34:1933–1972. doi: 10.1002/anie.199519331. [DOI] [Google Scholar]
- (9).Best MD, Zhang H, Prestwich GD. Inositol Polyphosphates, Diphosphoinositol Polyphosphates and Phosphatidylinositol Polyphosphate Lipids: Structure, Synthesis, and Development of Probes for Studying Biological Activity. Nat Prod Rep. 2010;27(10):1403–1430. doi: 10.1039/b923844c. [DOI] [PubMed] [Google Scholar]
- (10).Saleem H, Tovey SC, Rahman T, Riley AM, Potter BVL, Taylor CW. Stimulation of Inositol 1,4,5-Trisphosphate (IP3) Receptor Subtypes by Analogues of IP3. PLoS One. 2013;8(1):e54877. doi: 10.1371/journal.pone.0054877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Saleem H, Tovey SC, Riley AM, Potter BVL, Taylor CW. Stimulation of Inositol 1,4,5-Trisphosphate (IP3) Receptor Subtypes by Adenophostin A and Its Analogues. PLoS One. 2013;8(2):e58027. doi: 10.1371/journal.pone.0058027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Dohle W, Su X, Mills SJ, Rossi AM, Taylor CW, Potter BVL. A Synthetic Cyclitol-Nucleoside Conjugate Polyphosphate Is a Highly Potent Second Messenger Mimic. Chem Sci. 2019:5382–5390. doi: 10.1039/c9sc00445a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Thomas MP, Mills SJ, Potter BVL. The “Other” Inositols and Their Phosphates: Synthesis, Biology, and Medicine (with Recent Advances in Myo-Inositol Chemistry) Angew Chemie Int Ed. 2016;55:1614–1650. doi: 10.1002/anie.201502227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Taylor CW, Da Fonseca PCA, Morris EP. IP3 Receptors: The Search for Structure. Trends Biochem Sci. 2004;29(4):210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- (15).Safrany ST, Wojcikiewicz RJH, Strupish J, Nahorski SR, Dubreuil D, Cleophux J, Gero SD, Potter BVL. Interaction of Synthetic D-6-Deoxy-Myo-Inositol 1,4,5-Trisphosphate with the Ca2+- Releasing D-Myo-Inositol 1,4,5-Trisphosphate Receptor, and the Metabolic Enzymes 5-Phosphatase and 3-Kinase. FEBS Lett. 1991;278(2):252–256. doi: 10.1016/0014-5793(91)80128-p. [DOI] [PubMed] [Google Scholar]
- (16).Wilcox RA, Primrose WU, Nahorski SR, Challiss RAJ. New Developments in the Molecular Pharmacology of the Myo-Inositol 1,4,5-Trisphosphate Receptor. Trends Pharmacol Sci. 1998;19:467–475. doi: 10.1016/S0165-6147(98)01260-7. [DOI] [PubMed] [Google Scholar]
- (17).Saleem H, Tovey SC, Molinski TF, Taylor CW. Interactions of Antagonists with Subtypes of Inositol 1,4,5-Trisphosphate (IP3) Receptor. Br J Pharmacol. 2014;171(13):3298–3312. doi: 10.1111/bph.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Konieczny V, Stefanakis JG, Sitsanidis ED, Ioannidou N-AT, Papadopoulos NV, Fylaktakidou KC, Taylor CW, Koumbis AE. Synthesis of Inositol Phosphate-Based Competitive Antagonists of Inositol 1,4,5-Trisphosphate Receptors. Org Biomol Chem. 2016;14:2504–2514. doi: 10.1039/C5OB02623G. [DOI] [PubMed] [Google Scholar]
- (19).Li W, Schultz C, Llopis J, Tsien RY. Membrane-Permeant Esters of Inositol Polyphosphates, Chemical Syntheses and Biological Applications. Tetrahedron. 1997;53(35):12017–12040. doi: 10.1016/S0040-4020(97)00714-X. [DOI] [Google Scholar]
- (20).Conway SJ, Miller GJ. Biology-Enabling Inositol Phosphates, Phosphatidylinositol Phosphates and Derivatives. Nat Prod Rep. 2007;24:687–707. doi: 10.1039/b407701f. [DOI] [PubMed] [Google Scholar]
- (21).Li X, Gu C, Hostachy S, Sahu S, Wittwer C, Jessen HJ, Fiedler D, Wang H, Shears SB. Control of XPR1-Dependent Cellular Phosphate Efflux by InsP8 Is an Exemplar for Functionally-Exclusive Inositol Pyrophosphate Signaling. Proc Natl Acad Sci U S A. 2020;117(7):3568–3574. doi: 10.1073/pnas.1908830117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wilcox RA, Fauq AH, Kozikowski AP, Nahorski SR. Defining the Minimal Structural Requirements for Partial Agonism at the Type I Myo-Inositol 1,4,5-Trisphosphate Receptor. FEBS Lett. 1997;402(2–3):241–245. doi: 10.1016/S0014-5793(96)01540-2. [DOI] [PubMed] [Google Scholar]
- (23).Keddie NS, Ye Y, Aslam T, Luyten T, Bello D, Garnham C, Bultynck G, Galione A, Conway SJ. Development of Inositol-Based Antagonists for the D-Myo-Inositol 1,4,5-Trisphosphate Receptor. Chem Commun. 2011;47:242–244. doi: 10.1039/C0CC03003A. [DOI] [PubMed] [Google Scholar]
- (24).Mills SJ, Riley AM, Murphy CT, Bullock AJ, Westwick J, Potter BVL. Myo-Inositol 1,4,6-Trisphosphorothioate and Myo-Inositol 1,3,4-Trisphosphorothioate: New Synthetic Ca2+-Mobilising Partial Agonists at the Inositol 1,4,5-Trisphosphate Receptor. Bioorg Med Chem Lett. 1995;5(3):203–208. [Google Scholar]
- (25).Murphy CT, Riley AM, Mills SJ, Lindley CJ, Potter BVL, Westwick J. Myo-Inositol 1,4,6-Trisphorothioate and 1,3,6-Trisphosphorothioate: Partial Agonists with Very Low Intrinsic Activity at the Platelet Myo-Inositol 1,4,5-Trisphosphate Receptor. Mol Pharmacol. 2000;57:595–601. doi: 10.1124/mol.57.3.595. [DOI] [PubMed] [Google Scholar]
- (26).Bello D, Aslam T, Bultynck G, Slawin AMZ, Roderick HL, Bootman MD, Conway SJ. Synthesis and Biological Action of Novel 4-Position-Modified Derivatives of d-Myo-Inositol 1,4,5-Trisphosphate. J Org Chem. 2007;72:5647–5659. doi: 10.1021/jo070611a. [DOI] [PubMed] [Google Scholar]
- (27).Rossi AM, Riley AM, Tovey SC, Rahman T, Dellis O, Taylor EJA, Veresov VG, Potter BVL, Taylor CW. Synthetic Partial Agonists Reveal Key Steps in IP3 Receptor Activation. Nat Chem Biol. 2009;5(9):631–639. doi: 10.1038/nchembio.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Mills SJ, Luyten T, Erneux C, Parys JB, Potter BVL. Multivalent Benzene Polyphosphate Derivatives Are Non-Ca2+-Mobilizing Ins(1,4,5)P3 Receptor Antagonists. Messenger. 2012;2(1):167–181. doi: 10.1166/msr.2012.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Vandeput F, Combettes L, Mills SJ, Backers K, Wohlkonig A, Parys JB, De Smedt H, Missiaen L, Dupont G, Potter BVL, Erneux C. Biphenyl 2,3’,4,5’,6-Pentakisphosphate, a Novel Inositol Polyphosphate Surrogate, Modulates Ca2+ Responses in Rat Hepatocytes. FASEB J. 2007;21(7):1481–1491. doi: 10.1096/fj.06-7691com. [DOI] [PubMed] [Google Scholar]
- (30).Jenkins DJ, Potter BVL. A Ca2+-Mobilising Carbohydrate-Based Polyphosphate : Synthesis of 2-Hydroxyethyl α-D-Glucopyranoside 2',3,4-Trisphosphate. Carbohydr Res. 1996;287:169–182. doi: 10.1016/0008-6215(96)00078-x. [DOI] [PubMed] [Google Scholar]
- (31).Terauchi M, Abe H, Tovey SC, Dedos SG, Taylor CW, Paul M, Trusselle M, Potter BVL, Matsuda A, Shuto S. A Systematic Study of C-Glucoside Trisphosphates as Myo-Inositol Trisphosphate Receptor Ligands. Synthesis of β-C-Glucoside Trisphosphates Based on the Conformational Restriction Strategy. J Med Chem. 2006;49:1900–1909. doi: 10.1021/jm051039n. [DOI] [PubMed] [Google Scholar]
- (32).Shuto S, Tatani K, Ueno Y, Matsuda A. Synthesis of Adenophostin Analogues Lacking the Adenine Moiety as Novel Potent IP3 Receptor Ligands: Some Structural Requirements for the Significant Activity of Adenophostin A. J Org Chem. 1998;63:8815–8824. doi: 10.1021/jo980925l. [DOI] [Google Scholar]
- (33).Van Straten NCR, van der Marel GA, Van Boom JH. An Expeditious Route to the Synthesis of Adenophostin A. Tetrahedron. 1997;53(18):6509–6522. [Google Scholar]
- (34).Rosenberg HJ, Riley AM, Correa V, Taylor CW, Potter BVL. C-Glycoside Based Mimics of D-Myo-Inositol 1,4,5-Trisphosphate. Carbohydr Res. 2000;329:7–16. doi: 10.1016/s0008-6215(00)00175-0. [DOI] [PubMed] [Google Scholar]
- (35).Denis GV, Ballou CE. The Ca2+ Release Activities of D-Myo-Inositol 1,4,5-Trisphosphate Analogs Are Quantized. Cell Calcium. 1991;12(6):395–401. doi: 10.1016/0143-4160(91)90065-M. [DOI] [PubMed] [Google Scholar]
- (36).Rossi AM, Riley AM, Potter BVL, Taylor CW. Adenophostins: High-Affinity Agonists of IP3 Receptors. Curr Top Membr. 2010;66:209–233. doi: 10.1016/s1063-5823(10)66010-3. [DOI] [PubMed] [Google Scholar]
- (37).Shie C-R, Tzeng Z-H, Wang C-C, Hung S-C. Metal Trifluoromethanesulfonate Catalyzed Regioselective Reductive Ring Opening of Benzylidene Acetals. J Chin Chem Soc. 2009;56:510–523. doi: 10.1002/jccs.200900076. [DOI] [Google Scholar]
- (38).Bourdreux Y, Lemétais A, Urban D, Beau J-M. Iron(III) Chloride-Tandem Catalysis for a One-Pot Regioselective Protection of Glycopyranosides. Chem Commun. 2011;47:2146–2148. doi: 10.1039/c0cc04398b. [DOI] [PubMed] [Google Scholar]
- (39).Nurminen E, Lönnberg H. Mechanisms of the Substitution Reactions of Phosphoramidites and Their Congeners. J Phys Org Chem. 2004;17(1):1–17. doi: 10.1002/poc.681. [DOI] [Google Scholar]
- (40).Fylaktakidou KC, Duarte CD, Koumbis AE, Nicolau C, Lehn JM. Polyphosphates and Pyrophosphates of Hexopyranoses as Allosteric Effectors of Human Hemoglobin: Synthesis, Molecular Recognition, and Effect on Oxygen Release. ChemMedChem. 2011;6:153–168. doi: 10.1002/cmdc.201000366. [DOI] [PubMed] [Google Scholar]
- (41).Plante OJ, Andrade RB, Seeberger PH. Synthesis and Use of Glycosyl Phosphates as Glycosyl Donors. Org Lett. 1999;1(2):211–214. doi: 10.1021/ol9905452. [DOI] [PubMed] [Google Scholar]
- (42).Carrel FR, Seeberger PH. Protecting Group Manipulations on Glycosyl Phosphate Triesters. J Carbohydr Chem. 2007;26:125–139. doi: 10.1080/07328300701298204. [DOI] [Google Scholar]
- (43).Mills SJ, Rossi AM, Konieczny V, Taylor CW, Potter BVL. D-Chiro-Inositol Ribophostin: A Highly Potent Agonist at D-Myo-Inositol 1,4,5-Trisphosphate Receptors: Synthesis and Biological Activities. J Med Chem. doi: 10.1021/acs.jmedchem.9b01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Takahashi M, Kagasaki T, Hosoya T, Takahashi S. Adenophostins A and B: Potent Agonists of Inositol-1,4,5-Trisphosphate Receptor Produced by Penicillium Brevicompactum. J Antibiot (Tokyo) 1993;46(11):1643–1647. doi: 10.7164/antibiotics.46.1643. [DOI] [PubMed] [Google Scholar]
- (45).Rosenberg HJ, Riley AM, Laude AJ, Taylor CW, Potter BVL. Synthesis and Ca2+-Mobilizing Activity of Purine-Modified Mimics of Adenophostin A: A Model for the Adenophostin-Ins(1,4,5)P3 Receptor Interaction. J Med Chem. 2003;46:4860–4871. doi: 10.1021/jm030883f. [DOI] [PubMed] [Google Scholar]
- (46).Takahashi M, Tanzawa K, Takahashi S. Adenophostins, Newly Discovered Metabolites of Penicillium Brevicompactum, Act as Potent Agonists of the Inositol 1,4,5-Trisphosphate Receptor. J Biol Chem. 1994;269(1):369–372. [PubMed] [Google Scholar]
- (47).Fan G, Baker MR, Wang Z, Seryshev AB, Ludtke SJ, Baker ML, Serysheva II. Cryo-EM Reveals Ligand Induced Allostery Underlying InsP3R Channel Gating. Cell Res. 2018;28:1158–1170. doi: 10.1038/s41422-018-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Tegge W, Denis GV, Ballou CE. Synthesis and Ca2+-Release Activity of d- and l-Myo-Inositol 2,4,5-Trisphosphate and d- and l-Chiro-Inositol 1,3,4-Trisphosphate. Carbohydr Res. 1991;217:107–116. doi: 10.1016/0008-6215(91)84121-t. [DOI] [PubMed] [Google Scholar]
- (49).Riley AM, Unterlass JE, Konieczny V, Taylor CW, Helleday T, Potter BVL. A Synthetic Diphosphoinositol Phosphate Analogue of Inositol Trisphosphate. Med Chem Comm. 2018;9(7):1105–1113. doi: 10.1039/c8md00149a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Marchant JS, Beecroft MD, Riley AM, Jenkins DJ, Marwood RD, Taylor CW, Potter BVL. Disaccharide Polyphosphates Based upon Adenophostin A Activate Hepatic d-Myo-Inositol 1,4,5-Trisphosphate Receptors. Biochemistry. 1997;36:12780–12790. doi: 10.1021/bi971397v. [DOI] [PubMed] [Google Scholar]
- (51).Beecroft MD, Marchant JS, Riley AM, Van Straten NCR, Van Der Marel GA, Van Boom JH, Potter BVL, Taylor CW. Acyclophostin: A Ribose-Modified Analog of Adenophostin A with High Affinity for Inositol 1,4,5-Trisphosphate Receptors and PH-Dependent Efficacy. Mol Pharmacol. 1999;55(1):109–117. doi: 10.1124/mol.55.1.109. [DOI] [PubMed] [Google Scholar]
- (52).Safrany ST, Wilcox RA, Liu C, Dubreuil D, Potter BVL, Nahorski SR. Identification of Partial Agonists with Low Intrinsic Activity at the Inositol-1,4,5-Trisphosphate Receptor. Mol Pharmacol. 1993;43(4):499–503. [PubMed] [Google Scholar]
- (53).Riley AM, Murphy CT, Lindley CJ, Westwick J, Potter BVL. 6-Deoxy-6-Hydroxymethyl Scyllo-Inositol 1,2,4-Trisphosphate: A Potent Agonist at the Inositol 1,4,5-Trisphosphate Recpeptor. Bioorg Med Chem Lett. 1996;6(18):2197–2200. [Google Scholar]
- (54).Wilcox RA, Challiss RAJ, Traynor JR, Fauq AH, Ognayanov VI, Kozikowski AP, Nahorski SR. Molecular Recognition at the Myo-Inositol 1,4,5-Trisphosphate Receptor. J Biol Chem. 1994;269(43):26815–26821. [PubMed] [Google Scholar]
- (55).Fauq AH, Kozikowski AP, Ognayanov VI, Wilcox RA, Nahorski SR. Probing of the d-1,4,5-IP3/d-1,3,4,5-IP4 Functional Interface. Synthesis and Pharmacology of Novel d-3-Modified Myo-Inositol Trisphosphate Analogues. J Chem Soc, Chem Commun. 1994:1301–1302. [Google Scholar]
- (56).Burford NT, Nahorski SR, Chung S-K, Chang Y-T, Wilcox RA. Binding and Activity of the Nine Possible Regioisomers of Myo-Inositol Tetrakisphosphate at the Inositol 1,4,5-Trisphosphate Receptor. Cell Calcium. 1997;21(4):301–310. doi: 10.1016/s0143-4160(97)90118-4. [DOI] [PubMed] [Google Scholar]
- (57).Liu C, Potter BVL. Synthesis of 3-Position-Modified Analogues of Myo-Inositol 1,4,5-Trisphosphate, Tools for Investigation of the Polyphosphoinositide Pathway of Cellular Signaling. J Org Chem. 1997;62:8335–8340. doi: 10.1021/jo970926y. [DOI] [PubMed] [Google Scholar]
- (58).Safrany ST, Wilcox RA, Liu C, Potter BVL, Nahorski SR. 3-Position Modification of Myo-Inositol 1,4,5-Trisphosphate: Consequences for Intracellular Ca2+ Mobilisation and Enzyme Recognition. Eur J Pharmacol. 1992;226:265–272. doi: 10.1016/0922-4106(92)90071-3. [DOI] [PubMed] [Google Scholar]
- (59).Liu C, Nahorski SR, Potter BVL. Synthesis from Quebrachitol of 1L-Chiro-Inositol 2,3,5-Trisphosphate, an Inhibitor of the Enzymes of 1d-Myo-Inositol 1,4,5-Trisphosphate Metabolism. Carbohydr Res. 1992;234:107–115. [Google Scholar]
- (60).Hirata M, Watanabe Y, Yoshida M, Koga T, Ozaki S. Roles for Hydroxyl Groups of d-Myo-Inositol 1,4,5-Trisphosphate in the Recognition by Its Receptor and Metabolic Enzymes. J Biol Chem. 1993;268(26):19260–19266. [PubMed] [Google Scholar]
- (61).Dreef CE, Schiebler W, van der Marel GA, van Boom JH. Synthesis of 5-Phosphonate Analogues of Myo-Inositol 1,4,5-Trisphosphate: Possible Intracellular Calcium Antagonists. Tetrahedron Lett. 1991;32(42):6021–6024. [Google Scholar]
- (62).Lampe D, Liu C, Potter BVL. Synthesis of Selective Non-Ca2+ Mobilizing Inhibitors of D-Myo-Inositol 1,4,5-Trisphosphate 5-Phosphatase. J Med Chem. 1994;37(7):907–912. doi: 10.1021/jm00033a007. [DOI] [PubMed] [Google Scholar]
- (63).Li C-W, Dong H-J, Cui C-B. The Synthesis and Antitumor Activity of Twelve Galloyl Glucosides. Molecules. 2015;20:2034–2060. doi: 10.3390/molecules20022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Medgyes A, Farkas E, Lipták A, Pozsgay V. Synthesis of the Monosaccharide Units of the O-Specific Polysaccharide of Shigella Sonnei. Tetrahedron. 1997;53(12):4159–4178. doi: 10.1016/S0040-4020(97)00145-2. [DOI] [Google Scholar]
- (65).Tseberlidis G, Zardi P, Caselli A, Cancogni D, Fusari M, Lay L, Gallo E. Glycoporphyrin Catalysts for Efficient C-H Bond Aminations by Organic Azides. Organometallics. 2015;34:3774–3781. doi: 10.1021/acs.organomet.5b00436. [DOI] [Google Scholar]
- (66).D’Alonzo D, Guaragna A, Napolitano C, Palumbo G. Rapid Access to 1 , 6-Anhydro-l-Hexopyranose Derivatives via Domino Reaction: Synthesis of l-Allose and l-Glucose. J Org Chem. 2008;73:5636–5639. doi: 10.1021/jo800775v. [DOI] [PubMed] [Google Scholar]
- (67).Daragics K, Szabó P, Fügedi P. Some Observations on the Reductive Ring Opening of 4,6-O-Benzylidene Acetals of Hexopyranosides with the Borane Trimethylamine-Aluminium Chloride Reagent. Carbohydr Res. 2011;346:1633–1637. doi: 10.1016/j.carres.2011.04.046. [DOI] [PubMed] [Google Scholar]
- (68).Satomura S, Iwata T, Sakata Y, Omichi K, Ikenaka T. Synthesis of P-Nitrophenyl 6-O-Benzyl-α-Maltopentaoside, a Substrate for α-Amylases. Carbohydr Res. 1988;176:107–115. doi: 10.1016/0008-6215(88)84062-X. [DOI] [PubMed] [Google Scholar]
- (69).Bauder C. A Convenient Synthesis of Orthogonally Protected 2-Deoxystreptamine (2-DOS) as an Aminocyclitol Scaffold for the Development of Novel Aminoglycoside Antibiotic Derivatives against Bacterial Resistance. Org Biomol Chem. 2008;6:2952–2960. doi: 10.1039/b804784g. [DOI] [PubMed] [Google Scholar]
- (70).Boettcher S, Matwiejuk M, Thiem J. Acceptor-Influenced and Donor-Tuned Base-Promoted Glycosylation. Beilstein J Org Chem. 2012;8:413–420. doi: 10.3762/bjoc.8.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Liau BB, Milgram BC, Shair MD. Total Syntheses of HMP-Y1, Hibarimicinone, and HMP-P1. J Am Chem Soc. 2012;134(40):16765–16772. doi: 10.1021/ja307207q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Phillippy BQ, Bland JM. Gradient Ion Chromatography of Inositol Phosphates. Anal Biochem. 1988;175:162–166. doi: 10.1016/0003-2697(88)90374-0. [DOI] [PubMed] [Google Scholar]
- (73).Leatherbarrow RJ. GraFit Version 7. Erithacus Software Ltd; Horley, UK: 2009. Version 7. [Google Scholar]
- (74).Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE, II, Van Petegem F, Yule DI. Defining the Stochiometry of Inositol 1,4,5-Trisphosphate Binding Required to Initiate Ca2+ Release. Sci Signal. 2016;9(422):566–584. doi: 10.1126/scisignal.aad6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Guo F, Chiang MY, Wang Y, Zhang YZ. An in Vitro Recombination Method to Convert Restriction- and Ligation-Independent Expression Vectors. Biotechnol J. 2008;3(3):370–377. doi: 10.1002/biot.200700170. [DOI] [PubMed] [Google Scholar]
- (76).Laude AJ, Tovey SC, Dedos SG, Potter BVL, Lummis SCR, Taylor CW. Rapid Functional Assays of Recombinant IP3 Receptors. Cell Calcium. 2005;38:45–51. doi: 10.1016/j.ceca.2005.04.001. [DOI] [PubMed] [Google Scholar]
- (77).Tovey SC, Sun Y, Taylor CW. Rapid Functional Assays of Intracellular Ca2+ Channels. Nat Protoc. 2006;1(1):259–263. doi: 10.1038/nprot.2006.40. [DOI] [PubMed] [Google Scholar]
- (78).Cheng Y-C, Prusoff WH. Relationship between the Inhibition Constant (KI) and the Concentration of Inhibitor Which Causes 50 Per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- (79).Colquhoun D. Lectures on Biostatistics: An Introduction to Statistics with Applications in Biology and Medicine. Oxford University Press; London, UK: 1971. [Google Scholar]
- (80).Ott RL, Longnecker M. An Introduction to Statistical Methods and Data Analysis. Cengage Learning; Boston, USA: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.