Abstract
Objective:
While maternal folate deficiency has been linked to poor pregnancy outcomes such as neural tube defects, anemia, and low birth weight, the relationship between folate and preterm birth (PTB) in the context of US post-folic acid fortification era is inconclusive. We sought to explore the relationship between maternal folate status and PTB and its subtypes- spontaneous and medically indicated PTB.
Design:
observational study
Setting:
Boston Birth Cohort, a predominantly urban, low income, race-ethnic minority population at a high-risk for PTB.
Subjects:
7675 mother-infant dyads enrolled in the Boston Birth Cohort. A subsample (n=2,313) of these dyads had maternal plasma folate samples collected 24–72 hours after delivery.
Results:
Unadjusted and adjusted logistic regressions revealed an inverse relationship between the frequency of multivitamin supplement intake and PTB. Compared to less frequent use, multivitamin supplement intake 3–5 times/week (adjusted odds ratio (aOR)= 0.78, 95% confidence interval (CI): 0.64, 0.96) or >5 times/week (aOR= 0.77, 95% CI: 0.64, 0.93) throughout pregnancy was associated with reduced risk of PTB. Consistently, higher plasma folate levels (highest versus lowest quartile) were associated with lower risk of PTB (aOR= 0.74, 95% CI: 0.56, 0.97). The above associations were similar among spontaneous and medically indicated PTBs.
Conclusions:
If confirmed by future studies, our findings raise the possibility that optimizing maternal folate levels across pregnancy may help to reduce the risk of PTB among the most vulnerable US population in the post-folic acid fortification era.
INTRODUCTION
Preterm birth (PTB, birth before 37 completed weeks of gestation) has been recognized as one of the most pressing challenges to maternal and child health in the United States and the world (1). The role of maternal nutrition remains a promising but understudied area of investigation in the identification of important and modifiable risk factors for PTB.
Folates are a group of naturally occurring water-soluble B vitamins involved in biological reactions needed for fetal and placental growth such as DNA synthesis, repair and methylation (2). Maternal folate deficiency is a modifiable nutritional status that has been linked with adverse pregnancy outcomes such as neural tube defects, congenital anomalies, low birthweight, maternal megaloblastic anemia and preeclampsia (3–5). Folic acid is a synthetic form of folate that is used in multivitamin supplements and grain product fortification. Despite the establishment of national folate intake recommendations and mandatory folic acid fortification programs in the US since 1998 (6, 7), folate consumption is still of public health significance as data suggests that 25% of women of reproductive age have insufficient folate levels (8).
To date, much attention has been paid to the role of periconception folate intake to prevent neural tube defects (NTDs) in offspring (a first trimester event) (9–12). However, considerable knowledge gaps remain regarding the role of folate in PTB (a third trimester event). A population-based analysis demonstrated reduced PTB risk after the implementation of mandatory folic acid fortification in US (13). Some US studies have found an association between lower folate status and PTB, (14–18) while other studies found no association (19, 20). These mixed results may be due to variations across studies in terms of sociodemographic characteristics of the study population, whether the studies were conducted prior to or after the mandatory folic acid fortification program, differences in definitions and measurement of folate status (self-reported intake versus biomarkers), and/or timing of folic acid administration (preconception versus specific trimesters). The optimal timing of folic acid intake in relation to PTB remains unclear i.e., whether there is a critical window of folic acid intake such as the periconception period as has been demonstrated in the folate-NTDs relationship versus during specific trimester of gestation, given preterm birth is a later event. To date, most relevant studies were based on self-report of folic acid supplementation, which is known to be imprecise and associated with large variation in plasma folate levels, a biomarker of folate nutritional status (21). There is a need for contemporary post folic-acid-fortification studies that examine the associations between folic acid intake as well as folate biomarkers and PTB and how the association between folate status and PTB varies by PTB sub types- spontaneous vs. medically indicated (induced) PTB in high-risk US populations.
Our study sought to address the aforementioned gaps in understanding of the association between maternal folate status and PTB in a large, predominantly urban low-income minority birth cohort in the US. Specifically, we examined the relationship between PTB and self-reported preconception (six months prior to pregnancy) and pregnancy multivitamin supplementation (during each trimester), as well as biomarker measures of maternal plasma folate at delivery. We also explored whether the associations differed for spontaneous vs. medically indicated PTB.
SUBJECTS AND METHODS
Study population
We analyzed data from the Boston Birth Cohort (BBC) study which commenced in 1998 and is ongoing to date (22, 23). Our dataset included, 8494 mother-infant dyads enrolled in the study from 1998 to 2014. The BBC is registered at https://clinicaltrials.gov/ct2/show/NCT03228875. The initial and continuation of the study protocol were approved by the institutional Review Boards of Boston University Medical Center and Johns Hopkins Bloomberg School of Public.
Mothers who delivered at the Boston Medical Center (BMC), which serves a predominantly low-income, minority, inner-city patient population, were recruited 24–72 hours after delivery while still hospitalized. Of note, the length of stay in hospitals after childbirth in the US is 2 days for vaginal delivery and 4 days for caesarean section (24, 25). Cases were defined as mother-infant dyads with singleton, live, low birthweight (LBW; <2,500 grams) or preterm infants (<37 weeks of gestation) regardless of birthweight. Controls were defined as mother-infant dyads with singleton, live, term infants with birthweight 2,500g or more. Of note, this paper specifically examines PTB versus. term birth, regardless of birthweight.
Data collection
After informed consent was obtained, the study staff collected the epidemiological data, clinical data and maternal venous blood and placental samples. Epidemiological data were collected within 24–72 hours postpartum via an in person maternal questionnaire interview. Clinical data were abstracted from medical records using a standardized form. Plasma folate levels were measured in a subsample of the maternal blood samples obtained within 24–72 hours postpartum from mothers who continued to receive care at BMC.
Definition of key variables
PTB was defined as delivery before 37 completed weeks of gestation. Gestational age was determined using an algorithm based on the first day of the last menstrual period and the results of early ultrasound (<20 weeks’ gestation), as previously published (23).
Multivitamin supplement intake was determined during the maternal interview based on responses to the following questions: “Did you take prenatal vitamins prescribed by your doctor?” and “Did you take any over-the-counter multivitamins?” during pre-pregnancy (6 months prior to conception), 1st trimester (day 1 to day 90 of pregnancy), 2nd trimester (day 91 to day 180 of pregnancy), 3rd trimester (day 181 of pregnancy to birth)? Response categories included: none, 1 time per week, 2 times per week, 3–5 times per week, and almost daily. Based on responses to these two questions, preconception multivitamin intake was dichotomized (none vs. any). Intake for each trimester as well as across all trimesters was divided into the following categories: none, 1–2 times per week, 3–5 times, almost daily. In the US, prenatal or over-the-counter multivitamins typically contain 800 or 400 micrograms of folic acid respectively and are to be taken daily, however majority of mothers enrolled in the cohort received prenatal care and were advised to take prenatal vitamins which contain 800 micrograms of folic acid (26). A continuous measure of overall multivitamin supplement intake across all trimesters (henceforth referred to as the “multivitamin supplement intake index”) was developed by adding multivitamin intake across the three trimesters to create a composite index of multivitamin supplement intake across pregnancy. For each trimester, frequency of intake was coded as none=0, 1–2 times per week=1, 3–5 times per week=3, almost daily=4. Thus, the composite index ranged from 0 to 12.
Plasma folate concentrations were measured using chemiluminescent immunoassay with diagnostic kits (Shenzhen New Industries Biomedical Engineering Co., Ltd. China) using a Beckman Coulter ACCESS Immunoassay System (Beckman-Coulter Canada, Mississauga, Canada) (27). Plasma folate levels were assessed as i) a continuous variable in nmol/L; ii) quartiles of plasma folate levels; and iii) categorizations per the World Health Organization (WHO) guidelines (folate deficiency/insufficiency (<13.5nmol/l); normal (13.5–45.3nmol/l) and elevated (> 45.3nmol/l)) (28).
Other covariates included sociodemographic factors such as: maternal age at delivery (<20, 20–34, 35+ years), maternal education (≤elementary, high school or ≥college), race/ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic and Other), marital status (unmarried versus married), parity (nulliparous versus multiparous), receipt of public assistance including: WIC (Women Infants and Children), food stamps, AFDC (Aid to Families with Dependent Children now known as Temporary Aid to Needy Families), housing assistance or fuel assistance (yes versus no) and maternal nativity (US born versus foreign born). Behavioral risk factors included alcohol use (never versus any), smoking status (never used, ever used, used in pregnancy) and stress (an indicator for mother’s report of life or pregnancy as being ‘very’ stressful). Biomedical factors from abstracted records included preeclampsia disorders (preeclampsia, eclampsia, gestational hypertension, hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome), maternal diabetes (presence of either gestational or pre-gestational diabetes), intrauterine infection/inflammation (presence of maternal fever or placenta pathology findings of villitis, deciduitis, chorioamnionitis, chorionitis, subchorionitis, funisitis, free membranitis) and prepregnancy BMI -from self-reported height and weight - was grouped into 4 categories: underweight (<18.5kg/m2), normal weight (18.5–24.9kg/m2), overweight (25–29.9kg/m2), and obesity (>30kg/m2).
Statistics
All analyses were conducted using STATA version 14 (College Station, TX: StataCorp LP). Preliminary data analysis was performed in the full sample (N= 7,576) of all enrolled women with multivitamin supplement intake data and plasma folate subsample (n=2313) of these women that received follow- up pediatric care at BMC. Chi-squared tests for categorical variables and t-tests for continuous variables were used to compare maternal characteristics by PTB status. Cronbach’s alpha was used to ensure the reliability of the scale “multivitamin supplement intake index” (Cronbach’s alpha=0.87). Unadjusted and adjusted logit regressions were used to graph the probability of PTB by plasma folate level. Crude and adjusted logistic regressions were used to explore the relationship between PTB and self-reported multivitamin supplement intake and plasma folate level. Supplemental analyses were conducted for the PTB subgroups of spontaneous and medically indicated PTB. All P-values in the analyses were two-sided and the Type I error rate was set at 0.05.
RESULTS
This study was based on a full sample of 7,576 women with complete multivitamin supplement intake information from six months before conception to the third trimester of pregnancy and a sub-sample (n=2,313) with plasma folate samples collected at delivery. Those included in the full sample and those who had plasma folate data had similar baseline characteristics, except for a higher proportion of non-Hispanic Black mothers and PTBs in the subsample.
Table 1 displays the maternal characteristics for the total study population and plasma folate subsample (maternal characteristics by PTB subtype are presented in Supplemental Table 1). In the full sample, 27% of women had a preterm delivery. Compared to women with term births, women with PTB were more likely to be Non-Hispanic Black, older, US-born, unmarried, cigarette smokers, alcohol consumers and report a very stressful life or pregnancy. These women were also likely to have had preeclampsia disorders, intrauterine infection/inflammation, diabetes mellitus and be obese/overweight.
Table 1:
Maternal Characteristics of Study Population (N=7, 576)
| Maternal Characteristics | Multivitamin Supplement sample | Plasma folate subsample | ||||||
|---|---|---|---|---|---|---|---|---|
| Term | PTB | Term | PTB | |||||
| N | % | N | % | N | % | N | % | |
| 5,507 | 73 | 2,069 | 27 | 1,593 | 69 | 720 | 31 | |
| Race/ethnicity | ||||||||
| Non-Hispanic Blacka | 2,764 | 50.2 | 1,083 | 52.3 | 1,133 | 71.1 | 555 | 77.1 |
| Non-Hispanic White | 645 | 11.7 | 275 | 13.3 | 65 | 4.1 | 27 | 3.8 |
| Hispanic | 1,614 | 29.3 | 536 | 25.9 | 323 | 20.3 | 115 | 16.0 |
| Other | 451 | 8.2 | 163 | 7.9 | 72 | 4.5 | 23 | 3.2 |
| Missing | 33 | 0.6 | 12 | 0.6 | 0 | 0.0 | 0 | 0.0 |
| Age in years | ||||||||
| <20 | 608 | 11.0 | 195 | 9.4 | 159 | 10.0 | 67 | 9.3 |
| 20–34 | 4,053 | 73.6 | 1,446 | 69.9 | 1,171 | 73.5 | 494 | 68.6 |
| 35+ | 813 | 14.8 | 416 | 20.1 | 263 | 16.5 | 159 | 22.1 |
| Missing | 33 | 0.6 | 12 | 0.6 | 0 | 0.0 | 0 | 0.0 |
| Nativity (US born) | ||||||||
| Foreign born | 3,403 | 61.8 | 1,118 | 54.0 | 970 | 60.9 | 377 | 52.4 |
| US born | 2,029 | 36.8 | 935 | 45.2 | 596 | 37.4 | 338 | 46.9 |
| Missing | 75 | 1.4 | 16 | 0.8 | 27 | 1.7 | 5 | 0.7 |
| Education | ||||||||
| Less than high school | 1,680 | 30.5 | 623 | 30.1 | 434 | 27.2 | 204 | 28.3 |
| High School/GED | 1,800 | 32.7 | 741 | 35.8 | 575 | 36.1 | 279 | 38.8 |
| Some/beyond College | 1,970 | 35.8 | 684 | 33.1 | 578 | 36.3 | 234 | 32.5 |
| Missing | 57 | 1.0 | 21 | 1.0 | 6 | 0.4 | 3 | 0.4 |
| Marital Status | ||||||||
| Married | 2,038 | 37.0 | 698 | 33.7 | 564 | 35.4 | 225 | 31.3 |
| Unmarried | 3,339 | 60.6 | 1,339 | 64.7 | 1,019 | 64.0 | 488 | 67.8 |
| Missing | 130 | 2.4 | 32 | 1.5 | 10 | 0.6 | 7 | 1.0 |
| Receipt of public assistance b | ||||||||
| No | 856 | 15.5 | 354 | 17.1 | 208 | 13.1 | 112 | 15.6 |
| Yes | 4,651 | 84.5 | 1,715 | 82.9 | 1,385 | 86.9 | 608 | 84.4 |
| Parity | ||||||||
| Multiparous | 3,119 | 56.6 | 1,180 | 57.0 | 933 | 58.6 | 425 | 59.0 |
| Nulliparous | 2,374 | 43.1 | 885 | 42.8 | 657 | 41.2 | 294 | 40.8 |
| Missing | 14 | 0.3 | 4 | 0.2 | 3 | 0.2 | 1 | 0.1 |
| Cigarette smoking | ||||||||
| Never | 4,449 | 80.8 | 1,534 | 74.1 | 1,321 | 82.9 | 545 | 75.7 |
| Ever | 376 | 6.8 | 175 | 8.5 | 114 | 7.2 | 73 | 10.1 |
| Continued in pregnancy | 611 | 11.1 | 336 | 16.2 | 145 | 9.1 | 99 | 13.8 |
| Missing | 17 | 1.3 | 24 | 1.2 | 13 | 0.8 | 3 | 0.4 |
| Alcohol consumption | ||||||||
| No | 4,858 | 88.2 | 1,782 | 86.1 | 1,431 | 89.8 | 644 | 89.4 |
| Yes | 474 | 8.6 | 218 | 10.5 | 124 | 7.8 | 67 | 9.3 |
| Missing | 175 | 3.2 | 69 | 3.3 | 38 | 2.4 | 9 | 1.3 |
| Stressc | ||||||||
| No | 4,404 | 80 | 1,544 | 74.6 | 1,270 | 79.7 | 535 | 74.3 |
| Yes | 1,078 | 19.6 | 516 | 24.9 | 317 | 19.9 | 183 | 25.4 |
| Missing | 25 | 0.5 | 9 | 0.4 | 6 | 0.4 | 2 | 0.3 |
| Body Mass Index categories | ||||||||
| Underweight | 227 | 4.1 | 97 | 4.7 | 62 | 3.9 | 27 | 3.8 |
| Normal | 2,494 | 45.3 | 859 | 41.5 | 701 | 44.0 | 269 | 37.4 |
| Overweight/obese | 2,367 | 43 | 973 | 47 | 745 | 46.8 | 386 | 53.6 |
| Missing | 419 | 7.6 | 140 | 6.8 | 85 | 5.3 | 38 | 5.3 |
| Preeclampsia disorders d | ||||||||
| No | 5,047 | 91.6 | 1,582 | 76.5 | 1,483 | 93.1 | 534 | 74.2 |
| Yes | 460 | 8.4 | 487 | 23.5 | 110 | 6.9 | 186 | 25.8 |
| Intrauterine infection/inflammation | ||||||||
| No | 4,371 | 79.4 | 1,557 | 75.3 | 1,361 | 85.4 | 560 | 77.8 |
| Yes | 690 | 12.5 | 432 | 20.9 | 169 | 10.6 | 151 | 21.0 |
| Missing | 446 | 8.1 | 80 | 3.9 | 63 | 4.0 | 9 | 1.3 |
| Diabetes Mellitus | ||||||||
| None | 5,162 | 93.7 | 1,850 | 89.4 | 1,498 | 94.0 | 632 | 87.8 |
| Gestational | 220 | 4.0 | 124 | 6 | 53 | 3.3 | 49 | 6.8 |
| Pre-gestational | 75 | 1.4 | 79 | 3.8 | 31 | 1.9 | 34 | 4.7 |
| Missing | 50 | 0.9 | 16 | 0.8 | 11 | 0.7 | 5 | 0.7 |
| Multivitamin supplement intake | ||||||||
| Preconception | 9 | |||||||
| None | 5,137 | 93.3 | 1,940 | 93.8 | 1,504 | 94.4 | 671 | 93.2 |
| Any | 370 | 6.7 | 129 | 6.2 | 89 | 5.6 | 49 | 6.8 |
Abbreviation: GED-General Equivalency Diploma; N-number; PTB-preterm birth; SD-standard deviation; US-United States; WHO-World Health Organization.
Non-Hispanic Black includes Black, African American, Haitian, Cape Verdian.
Public assistance is defined as receipt of any of the following: WIC (Women Infants and Children), food stamps, AFDC (Aid to families with dependent children), housing assistance or fuel assistance.
Mother’s self-report of life or pregnancy being very stressful.
Preeclampsia disorders are defined as the presence of preeclampsia, gestational hypertension and hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome
Within the plasma folate subsample, 31% of women experienced PTB. The mean (SD) and median plasma folate concentration at delivery was 32.3 (20.0) nmol/L and 28.5 nmol/L respectively for PTB, significantly lower than 36.3 (24.7) nmol/L and 31.5 nmol/L for term births. Significant associations between maternal characteristics and PTB that were seen in the total supplement intake sample persisted in the plasma folate subsample. Plasma folate was mildly correlated with multivitamin supplement intake in the third trimester (rho: 0.10, p<0.001). The relationship between plasma folate concentrations and gestational age is presented graphically supplemental Figure 1. Every unit increase in plasma folate concentration was associated with a 0.01 week increase in gestational age (95% CI: 0.004, 0.016).
Table 2 displays the association between self-reported multivitamin supplement intake and PTB (analysis by PTB subtypes is presented in Supplemental Table 2). The overall multivitamin supplement intake across pregnancy was significantly associated with PTB. Specifically, each unit increase in the multivitamin supplement intake index reduced the odds of PTB (aOR= 0.98, 95% CI:0.97, 0.99). As a categorical variable, consistent multivitamin supplement intake (3–5 times/week or >5 times/week) reduced the odds of PTB compared to no intake across pregnancy (aOR= 0.78, 95% CI: 0.64, 0.96; aOR= 0.77, 95% CI: 0.64, 0.93, respectively). The relationship between multivitamin supplement intake across different time points and PTB showed that intake during the preconception period did not reduce PTB odds. Intake during the first trimester (>5 times/week) was associated with a reduction in PTB odds (aOR= 0.85, 95% CI: 0.73, 0.98). During the third trimester, intake of 3–5 times/week and >5 times/week was associated with lower odds of PTB (aOR= 0.75, 95% CI: 0.63, 0.85; aOR= 0.74, 95% CI: 0.64, 0.87, respectively). There was no significant difference in the odds of PTB among women with consistent multivitamin supplement intake of one to two times/week throughout pregnancy compared to no intake throughout pregnancy. Sensitivity analysis presented in Supplemental Table 3 showed a consistent pattern of multivitamin supplement intake at all time points with PTB among non-Hispanic Blacks only.
Table 2:
Relationship Between Multivitamin Supplement Intake and Preterm Birth (N=7,576)
| Period | Term (N=5507) |
Preterm (N=2,069) |
Unadjusted | Adjusted ORa | ||||
|---|---|---|---|---|---|---|---|---|
| N of cases |
(%) | N of cases |
(%) | OR | 95% CI | OR | 95% CI | |
| Multivitamin supplement intake in pregnancy (1st to 3rd trimester) | ||||||||
| Continuous (index) b |
n/a | n/a | n/a | n/a | 0.97 | 0.96, 0.98 | 0.98 | 0.97, 0.99 |
| Categorical | ||||||||
| None (ref) | 436 | 7.9 | 219 | 10.6 | 1.00 | n/a | 1.00) | n/a |
| 1–2x a week | 933 | 16.9 | 406 | 19.6 | 0.87 | 0.71, 1.06 | 0.91 | 0.74, 1.12 |
| 3–5x a week | 1373 | 24.9 | 476 | 23.0 | 0.69 | 0.57, 0.84 | 0.78 | 0.64, 0.96 |
| >5x a week | 2765 | 50.2 | 968 | 46.8 | 0.70 | 0.58, 0.83 | 0.77 | 0.64, 0.93 |
| Multivitamin supplement intake in specific time periods | ||||||||
| Preconception | ||||||||
| None (ref) | 5137 | 93.3 | 1,940 | 93.8 | 1.00 | n/a | 1.00 | n/a |
| Any | 370 | 6.7 | 129 | 6.2 | 0.92 | 0.75, 1.14 | 0.90 | 0.72, 1.12 |
| First trimester | ||||||||
| None (ref) | 914 | 16.6 | 402 | 19.4 | 1.00 | n/a | 1.00 | n/a |
| 1–2x a week | 204 | 3.7 | 97 | 4.7 | 1.08 | 0.83, 1.41 | 1.08 | 0.82, 1.43 |
| 3–5x a week | 1582 | 26.9 | 539 | 26.1 | 0.83 | 0.71, 0.96 | 0.90 | 0.76, 1.06 |
| >5x a week | 2907 | 52.8 | 1,031 | 49.8 | 0.81 | 0.70, 0.92 | 0.85 | 0.73, 0.98 |
| Second trimester | ||||||||
| None (ref) | 642 | 11.7 | 290 | 14.0 | 1.00 | n/a | 1.00 | n/a |
| 1–2x a week | 220 | 4.0 | 108 | 5.2 | 1.09 | 0.83, 1.42 | 1.19 | 0.90, 1.57 |
| 3–5x a week | 1558 | 28.3 | 570 | 27.6 | 0.81 | 0.68, 0.96 | 0.92 | 0.77, 1.10 |
| >5x a week | 3087 | 56.1 | 1,101 | 53.2 | 0.79 | 0.68, 0.92 | 0.86 | 0.73, 1.02 |
| Third trimester | ||||||||
| None (ref) | 655 | 11.9 | 345 | 16.8 | 1.00 | n/a | 1.00 | n/a |
| 1–2x a week | 255 | 4.6 | 99 | 4.8 | 0.74 | 0.56, 0.96 | 0.80 | 0.61, 1.06 |
| 3–5x a week | 1558 | 28.3 | 543 | 26.2 | 0.66 | 0.56, 0.78 | 0.75 | 0.63, 0.90 |
| >5x a week | 3039 | 55.2 | 1,082 | 52.3 | 0.68 | 0.58, 0.78 | 0.75 | 0.64, 0.87 |
Abbreviations: CI-confidence interval; N-number, OR-odds ratio, ref: reference
Adjusted for maternal race, age, nativity, education, marital status, receipt of public assistance, parity, cigarette use, alcohol use, stress, body mass index (BMI), preeclampsia disorders, intrauterine infection/inflammation and diabetes mellitus.
Composite measure of supplement intake from 1st to 3rd trimester
Table 3 displays the unadjusted and adjusted odds of PTB by plasma folate concentration (analysis by PTB subtypes is presented in Supplemental Table 3). Each unit and interquartile increase in plasma folate concentration reduced the odds of PTB (aOR= 0.99, 95% CI: 0.99, 1.00; aOR= 0.88, 95% CI:0.79, 0.97, respectively). This association persisted when plasma folate concentration was categorized. Compared to the lowest quartile (<19.4 nmol/L), the highest quartile of plasma folate concentration (>43.8 nmol/L) was associated with an over 25% reduction in PTB odds (aOR= 0.72, 95% CI: 0.54, 0.94). Similarly, excess plasma folate concentration (>45.3 nmol/L) was associated with reduced odds of PTB (aOR= 0.74, 95% CI: 0.56, 0.97) compared with normal plasma concentration (13.5–45.3 nmol/L).
Table 3:
Relationship between maternal plasma folate levels and preterm birth (n=2313)
| Plasma folate sample | Term (n=1593) |
Preterm (n=720) |
Unadjusted | Adjusted ORa | ||||
|---|---|---|---|---|---|---|---|---|
| N of cases |
% | N of cases |
% | OR | 95% CI | OR | 95% CI | |
| Continuous plasma folate concentration(nmol/L) | ||||||||
| Each unit increase1 |
n/a | n/a | n/a | n/a | 0.991 | 0.986, 0.995 |
0.994 | 0.990, 0.999 |
| Each interquartile increase in folate level2 |
n/a | n/a | n/a | n/a | 0.81 | 0.73, 0.90 | 0.88 | 0.79, 0.97 |
| Quartiles of plasma folate concentration(nmol/L) | ||||||||
| Lowest quartile: 6.6 to 19.4 (ref) |
378 | 23.7 | 201 | 27.9 | 1.00 | n/a | 1.00 | n/a |
| Second quartile: 19.4– 30.0 |
386 | 24.2 | 192 | 26.7 | 0.94 | 0.73, 1.19 | 1.02 | 0.79, 1.33 |
| Third quartile: 30.0–43.8 |
393 | 24.7 | 185 | 25.7 | 0.89 | 0.69, 1.13 | 1.09 | 0.83, 1.42 |
| Highest quartile: 43.8–185.5 |
436 | 27.4 | 142 | 19.7 | 0.61 | 0.47, 0.79 | 0.74 | 0.56, 0.97 |
| WHO classification | ||||||||
| Insufficiency/deficiency: <13.5 |
155 | 9.7 | 82 | 11.4 | 1.07 | 0.80, 1.43 | 0.86 | 0.63, 1.18 |
| Normal: 13.5– 45.3 (ref) |
1030 | 64.7 | 508 | 70.6 | 1.00 | n/a | 1.00 | 1.00 (ref) |
| Excess: ≥45.3 | 408 | 25.6 | 130 | 18.1 | 0.65 | 0.52, 0.81 | 0.70 | 0.55, 0.89 |
Abbreviations: CI-confidence interval; N-number, OR-odds ratio, ref: reference, WHO: World Health Organization.
Adjusted for maternal race, age, nativity, education, marital status, receipt of public assistance, parity, tobacco use, alcohol use, stress, BMI, preeclampsia disorders, intrauterine infection/inflammation, and diabetes.
Mean (SD) for term and preterm births: 36.6 (24.7) and 32.3 (20.0) nmol/L respectively.
Median (interquartile range) for term and preterm births: 31.5 (20.8–45.6) and 28.5 (19.0–38.5) nmol/L respectively.
Figure 1 displays the association between plasma folate concentration at delivery and probability of PTB, stratified by subtypes. Plasma folate concentration demonstrated a mild curvilinear relationship with overall PTB, a linear relationship with spontaneous PTB, and a curvilinear relationship with medically indicated PTB where higher concentrations of plasma folate were associated with a reduced probability of PTB or its subtypes.
FIGURE 1:
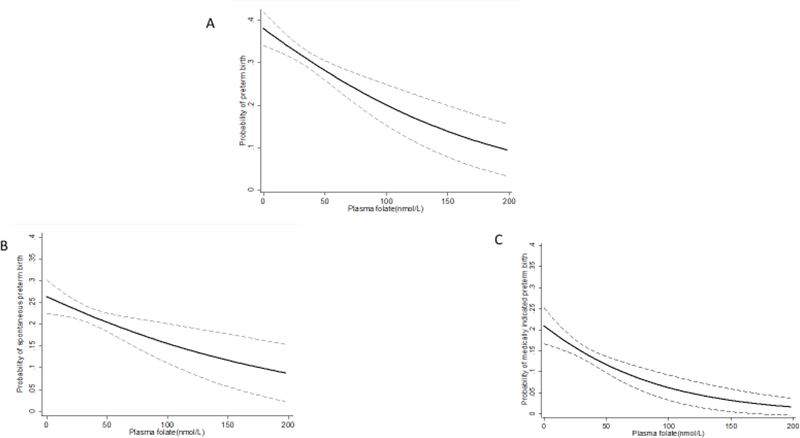
Probability of Overall (A), Spontaneous (B) and Medically Indicated (C) Preterm Birth by Plasma Folate Concentration
In Table 4, the relationship between plasma folate concentration and spontaneous versus medically indicted PTB is presented. The final regression model for medically indicated PTB did not include biomedical risk factors to avoid the introduction of factors potentially in the causal pathway. Each unit increase in plasma folate concentration was associated with reduced odds of spontaneous (aOR= 0.99, 95% CI: 0.99, 1.00) as well as medically indicated (aOR= 0.98, 95 % CI: 0. 0.98, 0.99) PTB. Among medically indicated preterm births, plasma folate concentration in the highest quartile was associated with a reduction in the odds of PTB; this relationship did not reach statistical significance for spontaneous PTB. Plasma folate concentrations greater than 45.3 nmol/L were associated with a 30% reduction in the odds of spontaneous (aOR: 0.72; 95% CI: 0.55, 0.95) and medically indicated PTB (aOR: 0.58; 95% CI: 0.40, 0.82).
Table 4:
Plasma folate levels and unadjusted and adjusted odds of PTB subtypes
| Spontaneous PTB | Medically indicated PTB | |||||||
|---|---|---|---|---|---|---|---|---|
| Plasma folate levels |
N of cases |
(%) | aOR a | 95% CI | N of cases |
(%) | aOR b | 95% CI |
| Continuous plasma folate concentration(nmol/L) | ||||||||
| Each unit increase |
n/a | n/a | 0.995 | 0.990, 1.000 |
n/a | n/a | 0.99 | 0.s98, 0.99 |
| Each interquartile increase in folate level |
n/a | n/a | 0.90 | 0.80,1.01 | n/a | n/a | 0.73 | 0.62, 0.87 |
| Quartiles of plasma folate concentration(nmol/L) | ||||||||
| Lowest quartile: 6.6–19.4 (reference) |
114 | 23.2 | 1.00 (ref) |
n/a | 87 | 18.7 | 1.00 (ref) |
n/a |
| Second quartile: 19.4–30.0 |
119 | 23.6 | 1.12 | 0.82, 1.53 | 73 | 15.9 | 0.85 | 0.60, 1.21 |
| Third quartile: 30.0–43.8 |
124 | 24.0 | 1.27 | 0.93, 1.73 | 61 | 13.4 | 0.68 | 0.47, 0.99 |
| Highest quartile: 43.8–185.5 |
96 | 18.1 | 0.86 | 0.62, 1.18 | 46 | 9.5 | 0.46 | 0.31, 0.69 |
| WHO classification (nmol/L) | ||||||||
| Insufficiency/deficiency: <13.5 |
45 | 22.5 | 0.77 | 0.53, 1.12 | 37 | 19.3 | 1.25 | 0.83, 1.86 |
| Normal: 13.5– 45.3 (reference) |
321 | 23.8 | 1.00 (ref) |
n/a | 187 | 15.4 | 1.00 (ref) |
n/a |
| Excess: ≥45.3 | 87 | 17.6 | 0.72 | 0.55, 0.95 | 43 | 9.5 | 0.58 | 0.40, 0.82 |
Abbreviation: aOR-adjusted odds ratio; n/a-not applicable; N-number; ref-reference; PTB: preterm birth; WHO-World Health Organization
Adjusted for maternal race, age, nativity, education, marital status, receipt of public assistance, parity, tobacco use, alcohol use, stress, BMI, preeclampsia disorders, intrauterine infection/inflammation, diabetes.
Adjusted for maternal race, age, nativity, education, marital status, receipt of public assistance, parity, tobacco use, alcohol use, stress
DISCUSSION
In our full sample, multivitamin supplement intake and plasma folate concentrations were generally adequate or high, as expected in this era of mandatory folic acid fortification of the food supply. Still, about a quarter of women had a relatively low plasma folate concentration (<19.4nmol/L), which was associated with an increased risk of PTB. While there is no national data, an association between folate fortification and reduced PTB rates was observed in California among live births that occurred from January 1990 through December 2000 (13). After adjusting for maternal age, parity, race/ethnicity, education, year of birth, and fortification period, fortification was shown to reduce PTB risk (relative risk ratios (RR) = 0.96; 95% CI 0.94, 0.97).
After controlling for confounding factors, our analysis shows that multivitamin supplement intake of at least 3 times/week throughout pregnancy was significantly associated with a reduction in the odds of PTB, consistent with other US based prospective studies that have assessed dietary folate intake (14, 16). A study in a low-income minority population showed that low (≤240μg/day) and intermediate (241–400μg/day) dietary folate intake were associated with an increased risk of PTB, respectively, as compared with women who had a folate intake >400μg/day (14). In another study, dietary folate intake ≤500μg was associated with an almost two times greater risk of preterm delivery (16).
We note that there was no significant association between preconceptional supplement intake and PTB, contrary to the findings of Bukowski et al., which demonstrated a 50%−70% related reduction in the incidence of early spontaneous PTB. However, in our study, preconception supplement intake was very low (7.1%) and may have resulted in reduced statistical power to detect significant associations. Multivitamin supplementation in the first and third trimester were both significantly associated with reduced PTB odds, with use during the third trimester associated with a greater reduction in PTB odds compared to use during the first trimester. It is unclear why second trimester multivitamin supplement intake is not associated with PTB. While the association observed in the third trimester may be a reflection of intake in other trimesters given the high correlation across trimesters, these findings suggest that the third trimester may be a critical time window in the folate-PTB relationship. While folate is needed for maternal tissue and fetal and placental growth throughout pregnancy, the rapid fetal development occurring during the third trimester is associated with maximum folate catabolism and thus increased requirements during this critical period (29, 30). Studies show that women who stopped multivitamin or folate supplementation after the first trimester had lower concentrations of maternal serum and red blood cell folate concentrations (29–31). The association between multivitamin supplement intake and PTB was corroborated using plasma folate concentration at delivery. Increasing plasma folate concentrations significantly reduced the odds of PTB. Our findings are consistent with other US-based studies with folate measurements during pregnancy wherein each 1 nmol/L increase in serum folate concentration at 28 weeks of gestation was also associated with reduced risk of PTB (14) and serum folate concentration less than 36.9 nmol/L in the second trimester (24–29 weeks of gestation) led to a nearly twofold increased risk of PTB (16). These findings demonstrating the relationship between maternal folate status and PTB are particularly important given that the national PTB rates have remained high, 10.4% in 2007, and 9.8% in 2016 despite research and intervention efforts (32). Our research on a predominantly minority population is also appropriate given that national PTB rates was highest among non-Hispanic black births (13.8%) (32). The patient population from which we derived the Boston Birth Cohort have a high rate of preterm birth (15–17%) and the higher rates of PTB seen in the study sample are because the study over-sampled preterm birth at the enrollment. Among this population at a particular high risk of preterm birth and inadequate folate intake, and we have shown that in such a setting, the lower levels of plasma folate with preterm delivery may be a reflection of the duration of folic acid supplementation and that maternal adequate folate status can reduce the risk of preterm birth (33).
Maternal factors well demonstrated in the literature to be associated with, but not necessarily in the causal pathway of PTB, include demographic, obstetric, medical, and psychosocial risk factors (34). However, the underlying mechanisms for the link between folate and PTB are not well-understood, but appear to be biologically plausible. For example, variations in key genes involved in folate metabolism such as dihydro folate reductase (DHFR) and serine hydroxy-methyl transferase (SHMT1) appear to increase the risk for spontaneous PTB (35). Other mechanistic pathways that may explain the folate – PTB relationship include hyperhomocysteinemia, placental implantation and intrauterine infection and inflammation (36, 37). Low folate status is associated with hyperhomocysteinemia, which has been linked with increased arterial stiffness, insulin resistance and endothelial dysfunction. Folate may also affect placental implantation and vascular remodeling through its role as a superoxide scavenger in antioxidant defenses (36). Folate deficiency is also associated with abnormal inflammatory responses, which could conceivably trigger premature parturition in the context of intrauterine infection (38, 39).
Strengths of our study include the use of complementary measures of folate status- from maternal self-report (capturing pattern of long-term use) and more objective biomarkers, providing multi-measure consistent evidence to support the folate-PTB relationship. The study is the largest investigation with recent birth cohort data on plasma folate and PTB published to date and is the first one that performed PTB subtype analyses. While maternal folate status has been linked with spontaneous PTB (18), its relationship with medically indicated PTB has only been demonstrated in animal studies (40). Again, such research is particularly relevant among high risk populations such as Non-Hispanic Blacks which have lower folate levels compared to other race/ethnic groups (8) and higher proportion of medically indicated PTB (41).
Our study contributes new knowledge to the field by exploring specific patterns of multivitamin supplementation during the preconception period and across trimesters. This facilitates identifying the critical period over the course of pregnancy to reinforce adequate folate intake in order to reduce PTB. Finally, our study focuses on the high-risk non-Hispanic black US populations in need for interventions to address both PTB and lower folate status.
We, however, acknowledge some limitations of this study. While plasma or serum folate is a common inexpensive measurement used in clinical and research setting, it only reflects short term folate status within the past few days in contrast to red cell folate concentrations which measure long-term folate status. Thus, plasma folate concentration at delivery can only be used as a proxy for third trimester folate concentration (42). As shown in a previous publication(21), maternal self-reported multivitamin intake during 3rd trimester was positively associated with maternal plasma folate levels. While folate measurements during early pregnancy would be more ideal, plasma folate concentration at delivery can be used as a proxy for third trimester folate concentration. In addition, multivitamin supplement intake was based on self-report, which is subject to recall bias as well as the fact that there was no information on the actual dosage of folic acid consumed. Also, the determination of folate status based on the frequency of supplement intake may be incomplete since folate status may also be influenced by dietary intake of folate rich/fortified foods and other factors affecting folate metabolism. Due to the high correlations between multivitamin supplement intake across all trimesters (rho: 0.58– 0.85, p<0.001), further adjustments for intake during other trimesters were not conducted when we explored the associations in each trimester. This was an observational study enriched by PTB, and by its nature cannot enable causal inference (43) as unobserved or uncontrolled confounding remains a threat to validity. Additional detail on some of the confounding variables, such as the level of cigarette and alcohol consumption in each trimester would have been helpful. While no randomized controlled trial has been conducted in the US and is unlikely given the advantageous role of folate on pregnancy outcomes, study findings need to be confirmed in other nationally representative prospective longitudinal studies. This is a US urban low income population in a post-folate fortification context and caution is needed in generalizing study findings to other populations with different characteristics. We also acknowledge that ours is a high-risk (low income, minority, urban) US population in a post-folate fortification context and that our findings may be reproducible in other populations in other countries.
There are important implications to be gleaned from this study. The association between folate and PTB among this predominantly minority, urban low-income population is important as studies have shown that women who were non-white (Non-Hispanic Black and Hispanic women), aged 18–24 years, and had less than a high school education or had a household income of <$25,000 are the least likely to report daily consumption of a supplement containing folic acid (44). In addition, minority populations are less likely to have heard about folic acid, to know it can prevent birth defects, and to consume foods fortified with folic acid or take vitamins containing folic acid (45, 46).
Lastly, our study supports the importance of consistent folate intake throughout pregnancy to mitigate adverse pregnancy outcomes, including PTB (9, 29, 47). This study demonstrated minimal difference in PTB mitigation related to multivitamin supplement intake of 3–5 times/week versus >5 times/week, suggesting a possible threshold dosing schedule of 3 times/week. If corroborated by other studies, this finding may impact the recommendations for frequency of multivitamin supplement intake before and during pregnancy. Specifically, this finding suggests that the same protective benefit can be derived from a 3 times weekly dose compared to a daily dose. Finally, folate has a broad biological function, and there is increasing recognition that folate supplementation during pregnancy may affect both short-term and long-term health of the offspring. For example, in the same cohort, we demonstrated beneficial effects of adequate maternal plasma folate levels on offspring obesity (48, 49). Furthermore, our recent study (21) along with that of others (50) raised concern about the potential risk of extremely high levels of folate on autism. Therefore, more work remains to be done to determine optimal range of maternal folate levels throughout pregnancy for major organs and systems in the offspring. Ultimately, we need to define an optimal range of folate levels (neither too low nor too high) preconception and during pregnancy, which can maximize its health benefits and minimize its risk. This may require careful consideration of a woman’s health conditions, dietary intake and folic acid supplementation, and measurement of plasma folate levels as needed.
Supplementary Material
Acknowledgements
Financial support: The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605); the National Institutes of Health (NIH) grants (R21ES011666, R01HD041702, R21HD066471, U01AI090727, R21AI079872, and R01HD086013); and the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) (R40MC27443, UJ2MC31074). We are also grateful for the general support from Hopkins Population Center (NICHD R24HD042854). This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by any funding agencies.
Footnotes
Ethical Standards Disclosure
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by Institutional Review Boards of Boston University Medical Center and the Johns Hopkins Bloomberg School of Public Health. Written informed consent was obtained from all subjects/patients.
Conflict of Interest
All authors have no conflicts of interest to disclose.
REFERENCES
- 1.Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Seminars in Fetal and Neonatal Medicine. 2016;21(2):68–73. [DOI] [PubMed] [Google Scholar]
- 2.Bailey LB, Gregory JF 3rd. Folate metabolism and requirements. The Journal of nutrition. 1999;129(4):779–82. [DOI] [PubMed] [Google Scholar]
- 3.Smits LJ, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. The Lancet. 2001;358(9298):2074–7. [DOI] [PubMed] [Google Scholar]
- 4.Zhu BP. Effect of interpregnancy interval on birth outcomes: findings from three recent US studies. International Journal of Gynecology & Obstetrics. 2005;89, Supplement 1:S25–S33. [DOI] [PubMed] [Google Scholar]
- 5.Moussa HN, Hosseini Nasab S, Haidar ZA et al. Folic acid supplementation: what is new? Fetal, obstetric, long-term benefits and risks. Future Science OA. 2016;2(2):FSO116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeiffer CM, Johnson CL, Jain RB et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. The American journal of clinical nutrition. 2007;86(3):718–27. [DOI] [PubMed] [Google Scholar]
- 7.Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinker SC, Hamner HC, Qi YP et al. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth defects research Part A, Clinical and molecular teratology. 2015;103(6):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czeizel AE, Dudas I, Vereczkey A et al. Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients. 2013;5(11):4760–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blom HJ. Folic acid, methylation and neural tube closure in humans. Birth Defects Res A Clin Mol Teratol. 2009;85(4):295–302. [DOI] [PubMed] [Google Scholar]
- 11.Smithells RW, Sheppard S, Schorah CJ et al. Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet (London, England). 1980;1(8164):339–40. [DOI] [PubMed] [Google Scholar]
- 12.Bibbins-Domingo K, Grossman DC, Curry SJ et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. Jama. 2017;317(2):183–9. [DOI] [PubMed] [Google Scholar]
- 13.Shaw GM, Carmichael SL, Nelson V et al. Occurrence of low birthweight and preterm delivery among California infants before and after compulsory food fortification with folic acid. Public Health Rep. 2004;119(2):170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholl TO, Hediger ML, Schall JI et al. Dietary and serum folate: their influence on the outcome of pregnancy. The American journal of clinical nutrition. 1996;63(4):520–5. [DOI] [PubMed] [Google Scholar]
- 15.Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. The American journal of clinical nutrition. 2000;71(5 Suppl):1295s–303s. [DOI] [PubMed] [Google Scholar]
- 16.Siega-Riz AM, Savitz DA, Zeisel SH et al. Second trimester folate status and preterm birth. American journal of obstetrics and gynecology. 2004;191(6):1851–7. [DOI] [PubMed] [Google Scholar]
- 17.Bodnar LM, Himes KP, Venkataramanan R et al. Maternal serum folate species in early pregnancy and risk of preterm birth. The American journal of clinical nutrition. 2010;92(4):864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukowski R, Malone FD, Porter FT et al. Preconceptional Folate Supplementation and the Risk of Spontaneous Preterm Birth: A Cohort Study. PLoS Medicine. 2009;6(5):e1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunlop AL, Taylor RN, Tangpricha V et al. Maternal Micronutrient Status and Preterm Versus Term Birth for Black and White US Women. Reproductive Sciences. 2012;19(9):939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw GM, Carmichael SL, Yang W et al. Periconceptional intake of folic acid and food folate and risks of preterm delivery. American journal of perinatology. 2011;28(10):747–52. [DOI] [PubMed] [Google Scholar]
- 21.Raghavan R, Riley AW, Volk H et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatric and perinatal epidemiology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surkan PJ, Dong L, Ji Y et al. Paternal involvement and support and risk of preterm birth: findings from the Boston birth cohort. Journal of psychosomatic obstetrics and gynaecology. 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zuckerman B, Pearson C et al. MAternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. Jama. 2002;287(2):195–202. [DOI] [PubMed] [Google Scholar]
- 24.Blumenfeld YJ, El-Sayed YY, Lyell DJ et al. Risk factors for prolonged postpartum length of stay following cesarean delivery. American journal of perinatology. 2015;32(9):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Development OfECa. Average length of stay: childbirth 2014/1 Paris: OECD Publishing; 2014. [ [Google Scholar]
- 26.Greenberg JA, Bell SJ. Multivitamin Supplementation During Pregnancy: Emphasis on Folic Acid and l-Methylfolate. Reviews in Obstetrics and Gynecology. 2011;4(3–4):126–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Huo Y, Li J, Qin X et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. (1538–3598 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 28.World Health O. Serum and red blood cell folate concentrations for assessing folate status in populations. 2015. [Google Scholar]
- 29.Wang S, Ge X, Zhu B et al. Maternal Continuing Folic Acid Supplementation after the First Trimester of Pregnancy Increased the Risk of Large-for-Gestational-Age Birth: A Population-Based Birth Cohort Study. Nutrients. 2016;8(8):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chanarin I, Rothman D, Ward A et al. Folate status and requirement in pregnancy. British Medical Journal. 1968;2(5602):390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNulty B, McNulty H, Marshall B et al. Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of Folic Acid Supplementation in the Second and Third Trimesters. The American journal of clinical nutrition. 2013;98(1):92–8. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton BE, Martin JA, Osterman MJ et al. Births: Provisional data for 2016. Vital Statistics Rapid Release. 2017;2. [Google Scholar]
- 33.Cheng TL, Mistry KB, Wang G et al. Folate Nutrition Status in Mothers of the Boston Birth Cohort, Sample of a US Urban Low-Income Population. American journal of public health. 2018;108(6):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler AS, Behrman RE, others. Preterm Birth:: Causes, Consequences, and Prevention: National Academies Press; 2007. [PubMed] [Google Scholar]
- 35.Johnson WG, Scholl TO, Spychala JR et al. Common dihydrofolate reductase 19-base pair deletion allele: a novel risk factor for preterm delivery. The American journal of clinical nutrition. 2005;81(3):664–8. [DOI] [PubMed] [Google Scholar]
- 36.Chen LW, Lim AL, Colega M et al. Maternal folate status, but not that of vitamins B-12 or B-6, is associated with gestational age and preterm birth risk in a multiethnic Asian population. The Journal of nutrition. 2015;145(1):113–20. [DOI] [PubMed] [Google Scholar]
- 37.Bergen N, Jaddoe V, Timmermans S et al. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG: An International Journal of Obstetrics & Gynaecology. 2012;119(6):739–51. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham F, Leveno K, Bloom S et al. Williams Obstetrics, 24e: McGraw-Hill; 2014. [Google Scholar]
- 39.Courtemanche C, Elson-Schwab I, Mashiyama ST et al. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. Journal of immunology (Baltimore, Md : 1950). 2004;173(5):3186–92. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M, Chen YH, Dong XT et al. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PloS one. 2013;8(12):e82713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19(12):773–82. [DOI] [PubMed] [Google Scholar]
- 42.Farrell C-JL, Kirsch SH, Herrmann M. Red cell or serum folate: what to do in clinical practice? Clinical chemistry and laboratory medicine. 2013;51(3):555–69. [DOI] [PubMed] [Google Scholar]
- 43.Szklo M, Nieto FJ. Epidemiology: beyond the basics: Jones & Bartlett Publishers; 2014. [Google Scholar]
- 44.Saccone G, Sarno L, Roman A et al. 5-Methyl-tetrahydrofolate in prevention of recurrent preeclampsia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015:1–5. [DOI] [PubMed] [Google Scholar]
- 45.Yang QH, Carter HK, Mulinare J et al. Race-ethnicity differences in folic acid intake in women of childbearing age in the United States after folic acid fortification: findings from the National Health and Nutrition Examination Survey, 2001–2002. The American journal of clinical nutrition. 2007;85(5):1409–16. [DOI] [PubMed] [Google Scholar]
- 46.Ahluwalia IB, Daniel KL. Are women with recent live births aware of the benefits of folic acid? MMWR Recomm Rep. 2001;50(RR-6):3–14. [PubMed] [Google Scholar]
- 47.Higgins JR, Quinlivan EP, McPartlin J et al. The relationship between increased folate catabolism and the increased requirement for folate in pregnancy. BJOG : an international journal of obstetrics and gynaecology. 2000;107(9):1149–54. [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Hu FB, Mistry KB et al. Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations With Child Metabolic Health. JAMA pediatrics. 2016;170(8):e160845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Mueller NT, Li J et al. Association of Maternal Plasma Folate and Cardiometabolic Risk Factors in Pregnancy with Elevated Blood Pressure of Offspring in Childhood. American Journal of Hypertension. 2017;30(5):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiens D, DeSoto M. Is High Folic Acid Intake a Risk Factor for Autism?—A Review. Brain Sciences. 2017;7(11):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


