Abstract
Background
The developmental period of adolescence marks the initiation of new socioemotional and physical behaviors, including sexual intercourse. However, little is known about neurodevelopmental influences on adolescent sexual decision-making.
Purpose
We sought to determine how subcortical brain volume correlated with condom use, and whether those associations differed by gender and pubertal development.
Methods
We used FreeSurfer to extract subcortical volume among N = 169 sexually experienced youth (mean age 16.07 years; 31.95% female). We conducted multiple linear regressions to examine the relationship between frequency of condom use and subcortical volume, and whether these associations would be moderated by gender and pubertal development.
Results
We found that the relationship between brain volume and condom use was better accounted for by pubertal development than by gender, and moderated the association between limbic brain volume and condom use. No significant relationships were observed in reward areas (e.g., nucleus accumbens) or prefrontal cortical control areas.
Conclusions
These data highlight the potential relevance of subcortical socioemotional processing structures in adolescents’ sexual decision-making.
Keywords: Condom use, Adolescence, Pubertal development, Gender, Magnetic resonance imaging (MRI)
Introduction
The transition to sexual intercourse is a normative, developmentally appropriate behavior often initiated during adolescence (1). Though not inherently risky, 40% of sexually active American youth engage in unprotected intercourse (2), which has very real consequences: 50% of new sexually transmitted infections (STIs) occur in this age group (3), and the rate of unintended pregnancies among 15–19 year olds is one of the highest in the nation (4). Safer sexual behavior requires cognitive and behavioral foresight to successfully enact protective steps to prevent unwanted pregnancies, as well as the acquisition of STIs, such as gonorrhea, chlamydia, and of most importance, the human immunodeficiency virus (HIV; (3)).
At this time, some of the best-supported adolescent STI/HIV prevention programs work for some, but not all youth, in instantiating long-term protective health behavior (e.g., consistent and correct condom use; (5, 6)). This has caused many to question the efficacy of these programs to improve condom use in this age group (e.g., (7)). We propose that one potential reason for the disconnect between STI/HIV risk reduction programming and adolescent health behavior change is that existing programs may not sufficiently access and engage the neurocognitive processes requisite for adolescent healthy sexual decision-making (e.g., (8)). Developmental neuroscience thus offers one avenue to pinpoint precisely which aspects of the developing brain may be relevant in adolescent sexual decision-making; data that is integral to guide future targeting of prevention programs to enhance response in relevant neurocognitive networks in this critical age group.
Neurodevelopment and Sexual Decision-Making
Adolescence is considered the second most important neurodevelopmental “sensitive period” after early childhood. During this period of growth, the brain reduces in overall volume, but improves in efficiency as pathways of connection and communication are established within the brain (a process termed “synaptic pruning”). Large-scale studies of adolescent brain development indicate that the timing and nature of brain development is not parallel between genders. Adolescent males, for example, have been found to have greater overall brain volume as compared to adolescent females; yet, adolescent females arrive at full brain maturation 1–2 years earlier than young males, who often do not complete frontal structure maturation until age ~15 frontal reward structure maturation until ~age 25 (9, 10–13). The result contributes to substantive shifts in subcortical emotional limbic (e.g., amygdala, hippocampus) and reward regions (e.g., nucleus accumbens), which subsequently catalyzes a reorientation of the adolescent brain toward peer-related social and emotional processing (14) and heightens social-emotional and reward learning (15).
Within the very limited body of published literature in the area of adolescent neurocognitive sexual decision-making, emerging studies are beginning to suggest that in contrast to other types of risk behavior (e.g., adolescent binge drinking) which may be driven, in large part, by the function and structure of brain frontal (control) regions (16), the arena of sexual risk increasingly suggests that the neural substrates underlying sexual decision-making may function in a very different way than other health risk behaviors in this age group (17–19). Moreover, these data seem to be centralized to limbic, rather than frontal, regions (19, 20).
Specifically, of the extant literature, emotional (limbic) centers, including the amygdala, and predominantly reward-based centers, including the nucleus accumbens, have been associated with sexual risk behavior (e.g., increased number of partners) in emerging adults (ages 18–22) (20). Critically, these data also suggested differences by gender, wherein the interaction between amygdala activation, ventral striatum activation (including the nucleus accumbens), and sexual risk was different for young males and females, with more activation in the amygdala associated with sexual risk for women, and less activation in the amygdala associated with sexual risk for men. In addition, functional examinations of brain response have highlighted the importance of the hippocampus within social feedback processing and social-emotional learning during this developmental period (21, 22). However, despite the growing body of functional neuroimaging studies implicating the relevance of subcortical limbic and reward regions in sexual decision-making, and despite potential differences by gender, we still know very little about how structural development of these regions relates to sexual decision-making in adolescence. Therefore, we aimed to explore potential relationships between risky sexual decision-making and brain volume in these subcortical regions implicated in social-emotional (amygdala, hippocampus) and reward processing (nucleus accumbens) in this age group; and further, examine these differences by gender.
Gender Differences in Brain Structure and Sexual Decision-Making
Along with emergent brain data around sexual risk, gender differences in adolescent sexual decision-making are also receiving increasing attention (23–25). This is relevant because in concert with observed differences in how young boys and girls negotiate sexual behavior, there are significant differences in the trajectory of neural maturation by gender (10), which impact limbic structures, including the amygdala and hippocampus. The result is that young males show relatively delayed maturation in important socioemotional areas, including the amygdala and hippocampus, as compared with young girls (26–28). These neurodevelopmental differences by gender are relevant, because they have been connected to other types of risk decision-making (29–31). Ultimately, despite the emerging role of the socioemotional areas during this period of development (32) and their potential implications in risk decision-making, we could find no examinations of adolescent brain structure and sexual risk behavior for human adolescents.
Pubertal Development of Brain Structure and Sexual Decision-Making
The emergence and course of pubertal development is another highly important biological factor that characterizes adolescent development (33), and varies by gender (34, 35). With regard to brain structure, pubertal development has a considerable impact on the development of subcortical structures (36), with testosterone and estradiol predicting limbic (amygdala) growth across adolescence (37). In terms of sexual risk behavior, earlier pubertal timing has been associated with earlier sexual debut, more frequent, and more risky sexual behavior (e.g., condomless intercourse, unwanted pregnancy, contracting STIs/HIV), especially among younger adolescent girls (38). Nevertheless, no studies to date have assessed the combined effects of pubertal development and neurodevelopment on risky sexual behavior. Further, it is standard procedure across many adolescent neuroimaging evaluations to collapse analyses across both gender and pubertal development. The result is an analytic approach that may miss subtle, but clinically relevant, developmental differences implicated in adolescent sexual risk decision-making.
The Current Study
The primary aim was to examine the relationship between brain structure and sexual decision-making in adolescents, and to assess whether these relationships might be moderated by gender and pubertal development. Commensurate with prior studies (17, 18), we operationalized safer sexual behavior as frequency of condom use. Given data suggesting the salient role of limbic, and potentially reward, regions in adolescent sexual decision-making (19, 20), we focused on examining key socioemotional (e.g., amygdala, hippocampus) and reward structures (e.g., nucleus accumbens) (e.g., (39, 40)), and conducted a postdoc examination of prefrontal structures (relevant for reward processing). We anticipated observing positive, linear associations between subcortical brain volumes for each region with condom use frequency, but no anticipated relationship between sexual risk behavior and prefrontal cortical control structures. Given the salience of gender differences in pubertal development (36), we anticipated observing the strongest associations for adolescents early in puberty and among boys more so than girls.
Method
Participants
All procedures were approved by the participating university institutional review board and with the additional protection of a federal Certificate of Confidentiality. Youth were recruited from justice-related programs in the southwest United States and completed magnetic resonance imaging (MRI) scanning as part of an ongoing study (41). The parent study was a randomized controlled trial examining two prevention programs to reduce adolescent STI/HIV risk (41); however, all data presented herein were collected prior to randomization to condition or exposure to prevention programming.
To recruit participants, trained research staff introduced the project at various collaborating programs, underscoring the voluntary nature of the study. Written assent was directly obtained from participants. Similar to other prevention studies (6), parent/guardian informed consent was obtained via telephone following youth assent. All consent conversations were audio-recorded and logged for proof of consent. In terms of inclusion criteria, youth had to be 14–18 years old, fluent in English, and actively participating in a justice-related program. Exclusion criteria included prescription for and/or taking antipsychotics/anticonvulsants, concussions or other head injuries leading to loss of consciousness for >5 min during the past 6 months, and other standard MRI contraindications (e.g., pregnancy) (42). In terms of enrollment, N = 280 youth assented/consented to participate in the study; of those, N = 253 successfully completed the neuroimaging procedures. Of those 253, N = 61 youth were removed from further analysis due to reporting no sexual activity. An additional N = 23 youth were removed from analyses due to having neuroimaging data that did not pass quality inspections. The sample thus contained N = 169 youth with a mean age of 16.07 years (standard deviation, SD = 1.17). All youth completed behavioral measures, followed by an MRI scan session. For this component, participants could earn between $20 and $70 depending on task performance during the functional MRI portion of the study (41).
Measures
Current Sexual Activity and Risky Sexual Behavior
Similar to other studies (5, 43), current sexual activity was assessed by querying whether adolescents had ever engaged in vaginal and/or anal intercourse (lifetime), defined by “a man putting his penis inside a woman’s vagina or inside someone’s anus (rear end)”. To assess risky sexual behavior, youth who endorsed engaging in sexual intercourse were queried about frequency of condom use (e.g., “In the past 3 months, how much of the time did you use condoms when you had sexual intercourse?”) measured on a 5-point scale (1 = Never, 2 = Almost never, 3 = Sometimes, 4 = Almost always, 5 = Always), and frequency of sexual intercourse, measured on a 6-point scale (1 = A few times a year, 2 = Once a month, 3 = Once a week, 4 = 2–3 times a week, 5 = 4–5 times a week, 6 = Almost every day).
Demographics
We queried age, self-reported race/ethnicity, gender, and highest educational attainment. To assess socioeconomic status, we evaluated: (i) parent education (1 = parent/guardian did not graduate from high school, 2 = parent/guardian graduated high school/got GED, 3 = parent/guardian some college but no degree, 4 = parent/guardian associates degree, 5 = parent/guardian bachelor’s degree, 6 = parent/guardian masters, PhD or MD) and (ii) family income, measured as youth eligibility for free lunch (1 = full price, 2 = reduced price, 3 = free).
Pubertal Development
The Pubertal Development Scale (44) is a self-report measure that asks participants to rate their stage of physical development across growth in height, body hair (underarm and pubic), and skin changes (pimples). Boys answered additional questions about voice deepening and facial hair, whereas girls answered additional questions about breast development and menstruation. All responses are on a Likert scale (1 = Not yet started, 2 = Barely started, 3 = Definitely underway, 4 = Seems completed) except for menstruation, which is coded as 1 = Premenarcheal or 4 = Postmenarcheal.
Image Acquisition and Statistical Analysis
Gray Matter Volume
High-resolution T1-weighted anatomic images were acquired on a 3 Tesla Siemens TIM Trio MRI scanner using a 12 channel head coil and the following 5-echo multiecho MPRAGE sequence: echo time (TE) 1.64/3.5/5.36/7.22/9.08 ms; repetition time (TR) 2,530 ms; voxel size 1 × 1 × 1 mm; 192 slices; field of view = 256 mm; acquisition time 6.03. Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite version 5.3 (http://surfer.nmr.mgh.harvard.edu/; see (45) for technical details). This study utilized the segmentation of the subcortical deep gray matter volumetric structures (hippocampus, amygdala, nucleus accumbens). All segmentation was visually inspected by two independent raters. Subjects with errors in the segmentation (including subjects with motion that would have affected segmentation) were excluded from analyses. Estimated volumes for the aforementioned regions in addition to total brain volume (from Freesurfer’s BrainSegVolNotVent), were extracted from the FreeSurfer output for each subject.
Analyses
All analyses were conducted using R 3.2.2 (46). Demographic and brain variables were examined for normality and subjected to Shapiro–Wilk tests. Homogeneity of variance was assessed using boxplots and Levene’s tests.
Gender, Pubertal Development, and Demographic/Risky Sexual Behavior Variables
As expected, most demographic and sexual risk variables were nonnormally distributed (Table 1). Mann–Whitney Rank Sum tests with Z values and Field’s r (sjmisc package in R; (47)) were utilized to examine whether demographic and sex risk variables significantly differed by gender and pubertal development. Chi-square and Cramér’s V effect sizes were used for categorical data (i.e., family income).
Table 1.
Subject demographics
| Demographics | % or M (SD) | % or M (SD) | Z value/χ2, p value | Effect size (Field’s r or Cramér’s V) | |
|---|---|---|---|---|---|
| Range | Girls (n = 54) |
Boys (n = 115) |
|||
| Ethnicitya | |||||
| Caucasian | 7% | 14% | |||
| African-American | 2% | 5% | |||
| Hispanic-American | 61% | 52% | |||
| American Indian/Alaskan Native | 9% | 2% | |||
| Multiethnic | 19% | 25% | |||
| Other | 2% | 1% | |||
| Age | 14–18 | 15.91 (1.20) | 16.14 (1.15) | −1.28, p = .20 | Field’s r = .10 |
| Family income (measured as free/discounted school lunch eligibility) | 1–3 | 2.35 (0.85) | 2.20 (0.89) | χ2=1.11, p = .58 | Cramér’s V = .08 |
| Maternal educationb | 1–6 | 2.26 (1.39) | 2.19 (1.12) | −0.21, p = .84 | Field’s r = .02 |
| Paternal educationc | 1–6 | 2.06 (1.39) | 2.12 (1.26) | −0.83, p = .41 | Field’s r = .07 |
| Last completed school grade | 5–12 | 9.06 (1.25) | 9.23 (1.17) | −1.13, p = .26 | Field’s r = .09 |
| Frequency of condom use | 1–5 | 3.31 (1.27) | 3.50 (1.24) | −1.01, p = .31 | Field’s r = .08 |
| Frequency of sexual intercourse | 1–6 | 2.72 (1.63) | 3.16 (1.52) | −1.86, p = .06 | Field’s r = .14 |
| Pubertal development scale score (PDS) | 2–4 | 3.55 (0.41) | 3.07 (0.46) | 6.02, p < .001 | Field’s r = .46 |
School lunch = how much paid for full school lunch on three point scale (1 = full price, 2 = reduced price, 3 = free); parental education = 6 point scale (1 = did not graduate from high school, 2 = graduated high school/got GED, 3= some college but no degree, 4 = associates degree, 5 = bachelor’s degree, 6 = masters, PhD or MD); frequency of condom use = 5 point scale (1 = Never, 5 = Always); frequency of sexual intercourse = 6 point scale (1 = A few times a year, 2 = Once a month, 3 = Once a week, 4 = 2–3 times a week, 5 = 4–5 times a week, 6 = Almost every day). M mean; SD standard deviation.
One male participant did not provide race/ethnicity data.
Two male participants missing data.
One female and five males missing data
Gender, Pubertal Development, and Subcortical Brain Volume
To examine gender and pubertal development with subcortical brain volume, we utilized independent samples t-tests. We began by comparing mean total brain volume from Freesurfer’s BrainSegVolNotVent, as well as raw subcortical volumes by gender, followed by regressions accounting for total brain volume in each volume comparison to correct for group differences in total brain volume. Regions of interest for subcortical volume assessment were the bilateral amygdala, hippocampus, and nucleus accumbens, with each hemisphere analyzed separately. We repeated these analyses replacing gender with mean-centered pubertal development.
Subcortical Brain Volume and Risky Sexual Behavior
To examine the relationship between targeted brain regions and risky sexual behavior, we utilized multiple regression. Bivariate correlations of the variables for possible inclusion in the regressions (gender, pubertal development, age, and total brain volume) were examined to aid in model selection (Table 2). Because of the high correlation between total brain volume and gender (r = .67), total brain volume deviation scores were used in lieu of the participants’ total brain volume values; these values were created by subtracting the appropriate gender-specific mean total brain volume from each participant’s total brain volume score, subsequently providing information about relative brain size unconfounded with gender (9). Other continuous independent variables (age, pubertal development, region of interest volumes) were mean-centered to aid in model interpretation. Gender was represented with weighted effects coding to account for disparate sample sizes.
Table 2.
Correlation matrix
| Total brain volume | Sex | Age | PDS | |
|---|---|---|---|---|
| TBVa | 1.00 | 0.67 | 0.08 | −0.25 |
| Sexb | 1.00 | 0.09 | −0.45 | |
| Age | 1.00 | 0.07 | ||
| PDSc | 1.00 |
Freesurfer’s BrainSegVolNotVent.
Boys = 1, Girls = 0.
Pubertal Development Scale (PDS; higher values = more pubertally developed).
Gender Versus Pubertal Development
In adolescent samples, gender and pubertal development can be inherently confounded, as girls progress through puberty earlier than boys (34, 35). Due to issues around multicollinearity, we examined the relative value of including both gender and pubertal development in the same regression model. Gender and pubertal development were first examined separately across each of the six regions of interest (right amygdala, left amygdala, right hippocampus, left hippocampus, right nucleus accumbens, left nucleus accumbens).
Full Models for Subcortical Brain Volume, Gender/Pubertal Development, and Risky Sexual Behavior
The relationship between each subcortical volume and condom use was examined controlling for age and total brain volume in two variations of a multiple regression model: Model 1 included gender as a main effect, plus a gender * region of interest interaction term, and Model 2 included pubertal development as a main effect, plus a pubertal development * region of interest interaction term. Model 3 included both interaction terms in the same model, in cases where both interactions were significant in Models 1 and 2.
Significant interaction terms in Model 3 were decomposed using simple slopes tests (pequod package in R; (48), which evaluated whether separate slopes for girls and boys (when decomposing gender moderation), or within a given high (+1 SD) or low (−1 SD) range of pubertal development scores (pubertal development moderation), were significantly different from zero when examining condom use frequency regressed on the subcortical region of interest.
Posthoc Examinations of Models Accounting for Intercourse Frequency, and Examination of the Relationship Between Prefrontal Cortex Volume and Risky Sexual Behavior
As findings from Models 1–3 could be driven, in part, by intercourse frequency, we added a mean-centered intercourse frequency variable to each of the three regression models, and replicated the process outlined above.
Due to the potential role of prefrontal cortical control regions in adolescent risk behavior, we conducted a posthoc examination of the potential relationship between prefrontal volumes and risky sexual behavior. In line with the models for examining relationships between risky sex and the subcortical volumes, we created a prefrontal cortex volume for each participant by combining Freesurfer-generated cortical volumes of the rostral middle frontal, caudal middle frontal, caudal anterior cingulate, and superior frontal regions across both hemispheres, in line with the approach taken by Mills and colleagues (49). Using the prefrontal cortex volume as the region of interest, we repeated the regression process outlined above, including intercourse frequency in each model.
Results
Gender, Pubertal Development, and Behavioral Variables
We found no significant gender differences in age, highest educational attainment, socioeconomic status, or risky sexual behavior (see Table 1). As expected, we observed gender differences on pubertal development, with girls showing significantly more advanced pubertal development than boys (p < .001, Field’s r = .46). Pubertal development was also significantly positively correlated with education (p < .05). No other relationships emerged for pubertal development across demographic/risky sexual behavior variables.
Gender and Subcortical Brain Volume
In this sample, overall total brain volume was significantly greater in boys than girls (p < .001, Table 3). Mean comparisons of raw subcortical volumes by gender also reflected significantly larger volumes in all but one examined region of interest for boys as compared with girls [bilateral hippocampus (p < .001); bilateral amygdala (p < .001); right nucleus accumbens (p < .01)]. No gender differences were observed for the left nucleus accumbens. When correcting for gender differences in the total brain volume, significant gender differences remained only for the bilateral nucleus accumbens [left nucleus accumbens (p < .01); right nucleus accumbens (p < .05)], and right amygdala (p < .05).
Table 3.
Subcortical volume comparisons by gender, with and without total brain volume correction
| No TBV correction |
TBV correction |
|||||
|---|---|---|---|---|---|---|
| Girls mean volume (SD) | Boys mean volume (SD) | tdf | CI (95%) | B | CI (95%) | |
| TBVa | 1,032,424 (79,084.61) | 1,187,703 (80,986.23) | t167= −11.71*** | −181460.3, −129097.4 | — | — |
| R Amyg | 1,346.45 (173.24) | 1,566.35 (161.66) | t167= −8.06*** | −273.77, −166.02 | 65.14* | 1.34, 128.94 |
| L Amyg | 1,436.24 (163.59) | 1,634.26 (163.45) | t167= −7.34*** | −251.27, −144.77 | 57.32 | −7.21, 121.85 |
| R Hipp | 4,000.64 (377.81) | 4,446.70 (413.43) | t167= −6.72*** | −577.14, −314.98 | 45.86 | −106.25, 197.97 |
| L Hipp | 4,004.59 (303.30) | 4,434.92 (404.88) | t134.9 = −7.69*** | −540.96, −319.71 | 75.54 | −68.91, 219.99 |
| R NAcc | 567.79 (86.65) | 606.04 (90.04) | t167 = −2.61** | −67.24, −9.28 | −42.32* | −76.96, −7.67 |
| L NAcc | 481.13 (107.24) | 484.04 (89.45) | t167 = −0.19 | −34.00, 28.17 | −63.73** | −102.96, −24.49 |
TBV (total brain volume) represented by Freesurfer’s BrainSegVolNotVent. Amyg amygdala; CI confidence interval; Hipp hippocampus; NAcc nucleus accumbens; SD standard deviation.
p < .05
p < .01
p < .001.
Pubertal Development and Subcortical Brain Volume
As expected, total brain volume was significantly inversely associated with pubertal development (p < .001, Table 4). Without correcting for total brain volume, we found a significant inverse relationship between brain volume and pubertal development across the right amygdala (p < .01), left amygdala (p < .05), right hippocampus (p < .01), and left hippocampus (p < .05). However, once total brain volume was controlled for, we observed no significant relationships between pubertal development and brain volume across our selected regions of interest.
Table 4.
Subcortical volume comparisons by pubertal development, with and without total brain volume correction
| No TBV correction |
TBV correction |
|||
|---|---|---|---|---|
| B | CI (95%) | B | CI (95%) | |
| TBVa | −55,240.00*** | −87,406.73, −23,073.86 | — | — |
| R Amyg | −88.60** | −146.81, −30.39 | −24.68 | −71.10, 21.73 |
| L Amyg | −57.61* | −114.58, −0.64 | 1.75 | −45.21, 48.71 |
| R Hipp | −199.70** | −335.37, −64.04 | −53.42 | −162.92, 56.08 |
| L Hipp | −139.91* | −268.88, −10.94 | −1.69 | −106.18, 102.81 |
| R NAcc | −14.41 | −42.15, 13.33 | 7.98 | −17.41, 33.37 |
| L NAcc | −8.31 | −37.55, 20.93 | 5.53 | −23.61, 34.68 |
TBV (total brain volume) represented by Freesurfer’s BrainSegVolNotVent. Amyg amygdala; CI confidence interval; Hipp hippocampus; NAcc nucleus accumbens.
p < .05
p < .01
p < .001.
Subcortical Brain Volume, Gender/Pubertal Development, and Risky Sexual Behavior
For Model 1, we examined the potential moderating effect of gender on the relationship between subcortical volume and condom use frequency. Here, we observed a significant gender * region of interest interaction in the bilateral hippocampus (p < .05), right amygdala (p < .05), and right nucleus accumbens (p < .05). For Model 2, we examined the potential moderating effect of pubertal development on the relationship between subcortical volume and condom use frequency. We observed a significant pubertal development * region of interest interaction in the bilateral hippocampus (p’s < .05), and bilateral amygdala (p’s < .05).
Since both interaction terms (i.e., gender * region of interest and pubertal development * region of interest) were significant for bilateral hippocampus, and the right amygdala, we then estimated Model 3 for those regions of interest. Model 3 included both two-way interaction terms and their accompanying main effect terms. For Model 3, we no longer observed a significant moderating effect of gender between examined brain volumes (e.g., bilateral hippocampus; right amygdala) and risky sexual behavior.
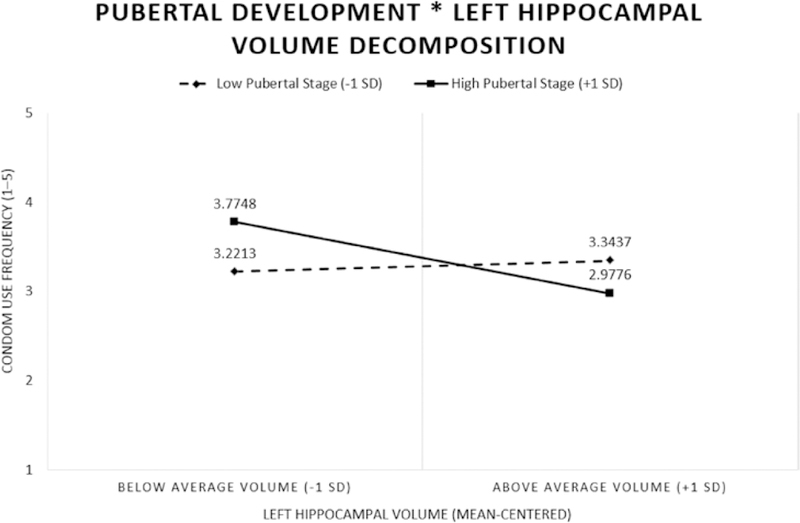
However, in Model 3, we did find that the pubertal development * region of interest interaction was significant in the bilateral hippocampus (e.g., right hippocampus (p < .05, 95% confidence interval [CI] [−0.002, −0.00001]); left hippocampus (p < .05, 95% CI [−0.002, −0.00002]), and right amygdala (p < .05, 95% CI [−0.004, −0.0001]). Follow up simple slopes tests of the pubertal development * region of interest interactions across the examined regions of interest (i.e., bilateral hippocampus, right amygdala) from Model 3 revealed significant slopes for the youth in more advanced pubertal status (pubertal development total, >1 SD) in the left hippocampus (p < .05, Fig. 1). Adolescents who were more pubertally advanced showed a significant negative relationship between condom use frequency and region of interest volume. In other words, for adolescents who were more sexually mature, those with a smaller left hippocampus reported using condoms more frequently. We did not observe any significant relationships between examined brain regions (e.g., bilateral hippocampus, right amygdala) and condom use frequency for the less pubertally developed youth (Table 5).
Fig. 1.
Pubertal development moderates the association between subcortical gray matter volume and condom use frequency in the left hippocampus. Adolescents more advanced in puberty (high pubertal development) showed a negative association between brain volume and condom use frequency.
Table 5.
Simples slopes for pubertal development * region of interest interaction terms
| Low pubertal development (−1 SD) | High pubertal development (+1 SD) | |
|---|---|---|
| R Amyg | B = 0.0009 | B = −0.0015 |
| R Hipp | B = 0.0005 | B = −0.0007 |
| L Hipp | B = 0.0001 | B = −0.0009* |
Amyg amygdala; Hipp hippocampus; SD standard deviation.
p< .05.
Posthoc Examination of Models Accounting for Intercourse Frequency
With the inclusion of intercourse frequency in Model 1, which examined the potential moderating effect of gender on the relationship between subcortical volume and condom use frequency, we observed a significant gender * region of interest interaction in the bilateral hippocampus (p < .05), bilateral amygdala (p < .05), and right nucleus accumbens (p < .05). For Model 2, which examined the potential moderating effect of pubertal development on the relationship between subcortical volume and condom use frequency, we observed a significant pubertal development * region of interest interaction in the bilateral hippocampus (right: p < .01; left: p < .05), and bilateral amygdala (p < .05). In other words, controlling for intercourse frequency did not alter observed results.
We estimated Model 3, accounting for intercourse frequency, for the bilateral hippocampus and the bilateral amygdala, since both the gender * region of interest and pubertal development * region of interest were significant in those four regions. We found no significant gender * region of interest interactions. We did find significant pubertal development * region of interest interactions in the bilateral hippocampus (p < .05 for each side; 95% CI for right hippocampus: [−0.002, −0.00005]; 95% CI for left hippocampus: [−0.002, −0.000003]), and left amygdala (p < .05; 95% CI [−0.005, −0.0007]). Simple slopes follow up examinations of these regions revealed significant slopes for the youth in more advanced pubertal status (pubertal development total, >1 SD) in the left hippocampus (p < .05) and the left amygdala (p < .05). Thus, even after accounting for intercourse frequency, more pubertally mature youth continued to show a negative relationship between brain volume and condom use frequency in these two regions. Model 3 (without intercourse frequency) showed significant interactions between PDS*ROI in bilateral hippocampus and right amygdala, but only the left hippocampus had significantly simple slopes. Model 3 (with intercourse frequency) showed significant interactions between PDS*ROI in bilateral hippocampus and left amygdala (instead of right amygdala), and the left hippocampus simple slopes finding replicated, plus the left amygdala.
Posthoc Analysis of Prefrontal Cortex Volume and Risky Sexual Behavior
For Model 1, we examined whether gender moderated the relationship between prefrontal cortex volume and condom use frequency after accounting for age, total brain volume, and intercourse frequency. There was no significant relationship between prefrontal cortex volume and condom use frequency, nor was there a significant interaction between prefrontal cortex volume and gender in the model. In Model 2, which included pubertal development in place of gender, there was again no significant relationship between prefrontal cortex volume and condom use frequency, nor a significant interaction between prefrontal cortex volume and pubertal status. Because the interaction terms in Models 1 and 2 were not significant, we did not proceed with Model 3.
Discussion
To our knowledge, this is the first empirical investigation of the relationship between subcortical brain structure and risky sexual behavior in a sample of sexually active adolescents. We expected to observe significant associations of socioemotional limbic (i.e., amygdala, hippocampus) and reward volumes (nucleus accumbens) with risky sexual behavior (condom use frequency) in adolescents. Further, we expected that these relationships would be moderated by gender and by pubertal development. We also expected these effects to be localized to socioemotional and reward, but not frontal control regions. In other words, we did not expect to observe any relationships between prefrontal cortical control volumes and risky sexual behavior. There was partial support for these hypotheses. We observed that gender and pubertal development directly predicted socioemotional limbic subcortical brain volume. This finding is highly in line with what we would expect, given gender differences in brain size and the natural process of pruning that occurs during adolescence (9).
Though it has been a source of debate, the current state of the neurodevelopmental literature has been to present adolescent brain data both with and without correction for total brain volume. We followed the recommendation of this field in presenting data both ways, which can help elucidate neurodevelopmental changes that might be obscured when examining the data either with, or without, this correction (9). Without total brain volume, we observed that boys had greater volumes in bilateral amygdala, bilateral hippocampus, and right nucleus accumbens. Further, in line with other studies of adolescent neurodevelopment (36), we observed significant differences in subcortical brain volume by pubertal development, with more developed youth showing smaller brain volumes across the bilateral amygdala relationship between pubertal development and reward (nucleus accumbens) volume. Further, in posthoc analyses, we found no relationship between pubertal development and/or risky sexual behaviors with prefrontal cortical control volumes.
Consistent with the broader literature (50), boys’ brains were overall significantly larger than the girls’ in this sample. When we corrected for natural differences in total brain volume, the effects were a bit more circumscribed, with significant gender differences only observed across the right amygdala and bilateral nucleus accumbens. With total brain volume controlled for, we no longer saw significant relationships between pubertal status and subcortical volumes. Ultimately, the alteration of the results once total brain volume is incorporated highlights the importance of examining subcortical brain volumes both with and without this form of correction, to reflect a comprehensive picture of the nature and location of what may be transpiring on a neural volumetric level in these types of neurodevelopment studies (9). Yet, these findings are strongly in line with the developmental health neuroscience literature in terms of nature and effect size (49, 51), and highlight the importance of continuing to examine these relationships in future work.
Although we originally observed a series of significant volume by gender interactions, we did not find evidence of gender as a significant moderator of the association between brain volume and risky sex above and beyond the contribution of pubertal development. Rather, our anticipated gender findings disappeared once pubertal development was added to the model. However, as predicted, pubertal development moderated the association between subcortical brain volumes and risky sexual behavior, and this was true even after accounting for frequency of sexual behavior (intercourse frequency). Interestingly, these associations were stronger for adolescents more advanced in pubertal development. One explanation for this finding may reflect potentially delayed neuromaturation, wherein the brains of youth with this significant moderated association between volume and risky sex may have yet to “catch up” with the rest of their body’s development. Our finding of associations later versus earlier in puberty also contrasts with other studies that indicate a stronger role for early puberty in adolescent sexual behavior (38). A second possibility is that subcortical volumes reflect maturational processes in sexual decision-making that are potentially occurring later in puberty, an approach that has generally not been assessed in adolescent sexual behavior studies.
Although the effect sizes may not seem overwhelming in the context of the adolescent health behavior literature, these are highly clinically meaningful differences in the field of neurocognitive perspectives on decision-making. Specifically, the specificity and involvement of key limbic regions (hippocampus, amygdala), but not reward and/or control structures, such as the nucleus accumbens and prefrontal cortex, suggests that socioemotional functioning rather than reward and/or control processing is critical in adolescent sexual decision-making (39, 40). Interestingly, the amygdala is at peak volume during adolescence, and represents one of the only brain regions that has direct receptors for sex hormones (52). Further, the amygdala is responsible for ascribing emotional significance to stimuli, subsequently influencing affective response and emotional learning (53). Relevant to the findings observed here, the amygdala interacts with the hippocampus; in concert, the hippocampus forms initial representation of the emotional significance of events, impacting the amygdala’s response to future related events (54). Thus, it is perhaps not surprising that in this sample, condom use in more pubertally mature adolescents was significantly associated with regions likely to be integral in pubertal development and timing, as well as in the neural communication around sexual drive, emotional valence, and the broader network of health decision-making.
Of further practical and clinical relevance, this pattern is distinct from what has been observed in the broader adolescent risk decision-making literature (55–57). More concretely, relationships of comparable effect size are generally observed in standard reward (e.g., nucleus accumbens) and prefrontal cortical control structures for other adolescent risk behaviors (e.g., substance use). However, these same statistical relationships were not observed between reward and/or control structures and adolescent sexual decision-making in this study. One reason for this might be that, even though the animal and adult literatures support the inherently rewarding nature of sexual activity (58, 59), reward might not be the sole, or most important, driver of sexual decision-making in this age group. Similarly, because of its relatively later emergence in adolescent cognitive development, many studies have implicated prefrontal cortical control systems as a primary driver of adolescent risk decision-making (60). However, a number of studies are beginning to take a more nuanced approach to the examination of behavioral control (32). In line with this recent movement in the field of adolescent risk decision-making, whereas an easy candidate for consideration in sexual decision-making, this study highlights that brain structures that drive prefrontal cortical control did not gain empirical support for their role in adolescent sexual decision-making here. Ultimately, risky sex is often collapsed into a broader group of adolescent risk behaviors (61); however, sexual decision-making may, in fact, be quite different from other types of adolescent health risk behavior. This is a finding of high clinical impact that calls for the need to carefully consider breaking the sexual risk neurocognitive literature away from the adolescent substance use neurocognitive literature. In fact, we submit that there might be several additional features that make adolescent sexual decision-making quite distinct from other types of adolescent risk behavior. For example, sexual behavior is neither teratogenic nor neurotoxic. In fact, it is requisite to the propagation of one’s own evolutionary line, as well as the overall species, and thus may be represented differently than less consequential behaviors (substance use, driving too fast, or skipping school) in the human brain.
Ultimately, these findings are particularly intriguing in light of behavioral studies showing different patterns of sexual decision-making by gender (62). The sample presented with gender differences in pubertal development. As such, the more physically developed group in the interaction decompositions may have a much greater proportion of females, due to natural differences in the timing of pubertal development by gender. To that end, these findings could still be capturing underlying gender differences. Other behavioral studies have reported that despite having more positive attitudes toward condoms, female adolescents and emerging adults are less likely than males to use condoms in practice (63). Further, behavioral studies show a strong role of socioemotional processing in adolescent sexual decision-making, with young men tending to consider reward, sexual pleasure, intimacy, and social development when making decisions about how and when to have sex (62, 64–67). In contrast, female youth are more likely to think about sex as a way to seek or achieve intimacy (65) and to improve or enhance relationship development or quality (68, 69). Taken together, these results highlight that relationship factors (70), potentially including individual goals for using condoms, and/or the pragmatic nature of negotiating heterosexual condom use (wherein males can unilaterally use a condom yet women must rely on or convince her partner to do so) may interfere with women’s condom use (62). Our volumetric differences across hippocampal and amygdala regions suggest that these salient socioemotional and affect-related prefrontal-amygdala and hippocampal circuits (71) may reflect a neural target for social and affective processing in adolescent cognition, communication, and negotiation, perhaps particularly for females, in sexual decision contexts. Related, these data contribute to an emerging body of research that suggests that although we might do well in terms of teaching young people about the practical benefits of using a condom, and feeling confident in their condom use, our current theoretical and empirically supported approaches may need to address neurodevelopmental findings to improve understanding of the emotional facets of this complex and inherently social behavior.
Strengths and Limitations
This study has a number of strengths, including a sample of sexually active youth and an empirical question that has been understudied in adolescent neurodevelopment and risk behavior (brain structure in the context of sexual health). At the same time, it is important to consider these findings in light of study limitations. First, some may view our sample as derived from a relatively unique population that would not generalize well to all adolescents. However, it is precisely this type of sample that is often missed in developmental neuroscience research (ethnically diverse, juvenile-justice involved youth). It is also important to highlight that more than 31 million youth are involved in the juvenile-justice system (72) and thus they represent an important population for study in their own right. Concretely, individuals within the clinical and scientific fields may believe that high risk, predominantly lower SES, Hispanic adolescents are somehow qualitatively different than the “general population” of adolescents. Though we can understand how it might be tempting to come to this conclusion, we respectfully disagree with this notion. Justice-involved adolescents like the ones in our sample are, in fact, much more mainstream than previously believed (73, 74). In several states within the USA, Hispanic youth represent the majority (75). More broadly, as of the 2010 US Census, minority youth are the majority in 10 states (76) will be the majority among all youth in the United States by 2020 (77). Nearly 20% of all adolescents live below the poverty line, and many more are from poor and low income families (nearly 50%) (78). Aside from the issue of race/ethnicity and socioeconomic status, researchers increasingly believe that far from being qualitatively different from their peers, youth involved in the juvenile-justice system simply represent the riskier end of a normal distribution of behavior (73). Thus, from our perspective, the adolescents in our study represent a critical, and often overlooked group within a normative continuum that intersects with and represents mainstream populations, but is often segregated in clinical and research settings. No data, including our own, supports making this distinction in any clinical or research population (79), and in fact, our lab is actively working to reduce this existing disparity in clinical and research efforts (75, 80, 81).
Second, given the wealth of changes in subcortical structures (amygdala, hippocampus) occurring in adolescence, it is necessary to further disentangle the independent contributions of pubertal development and gender on these brain-behavior relationships. In the current sample, girls were more advanced in pubertal development than boys, rendering it difficult to differentiate gender effects from puberty effects. Thus, future studies in this area would benefit from additional measures of pubertal development, such as Tanner ratings and hormonal levels, and a larger representation of pubertal stages across gender to clarify these relationships. A larger sample could facilitate the examination of the three-way interaction between gender, puberty, and region of interest, which the current study was under-powered to do. Third, we did not evaluate for co-occurring psychological disorders in this sample; future work would benefit from explicit inclusion of these co-occurring issues, as volumetric differences in these brain regions have been found in a number of psychological disorders and could potentially contribute to findings (82, 83).
Implications for Preventive Intervention
In terms of prevention programming, importantly, youth within this sample did not receive sexual health education through their juvenile-justice programming. Further, many of these youth were not routinely attending school, making it improbable that they received sex education in their academic setting; thus, these data are unlikely to be influenced by the receipt of external sex education programs that impacted their condom use prior to (or following) our brain and behavior measures.
In addition, these data suggest that different programs are potentially needed for physically more mature versus physically less mature adolescents (or potentially for young men versus young women), in order to effectively target programming to maximize behavioral response. This is congruent with prior work by our lab and others, which has shown that sexually less mature or sexually naive youth have very different expectations around condom use than youth who have already begun to engage in sexual behavior (62, 68). Further, recent work has suggested that we might have to begin developing targeted prevention programs to youth ages 10–12 if we want to reach them before they begin overt pubertal development (84). High-risk youth, particularly those involved in the juvenile-justice system, are often broken out by gender for the dissemination of sexual risk reduction programming, but they are not always broken out by age, pubertal status, and/or even sexual risk experience. Our data here suggest youth would potentially benefit from the articulation of targeted programming to the different cognitive and socioemotional developmental stages that occur during adolescence. We suggest that this presents a challenge to but also opportunity for the field of implementation science (85), which continues to gain momentum at this time.
In terms of specific articulation, in matching intervention content to neural mechanisms, this study highlights the role of socioemotional limbic systems, but not reward (nucleus accumbens) or control systems, in more pubertally developed youths’ sexual decision-making. Therefore, one avenue to consider in terms of treatment development for youth who are further along in pubertal development is to enhance program content that addresses socioemotional processing, especially in the context of dyadic decision-making, and when there is affection or love in the equation. Finally, one important caveat in this work is that we have focused on traditional delineations of gender and condomless penetrative sex as our risky sex metric. However, we realize that there are some limitations to this approach, particularly in terms of rapid advances in conceptualization of attraction, gender identity, sexual behavior, romantic relationship decision-making (86) and other forms of protected sex (e.g., PrEP/PEP) (87). These evolving conceptualizations will most certainly be explored in our future work.
Acknowledgements
This research was supported by the National Institutes of Health (NINR R01NR013332 to MPIs: Feldstein Ewing & Bryan). The funding source had no involvement in the collection, analysis, interpretation of data, the writing of the report, and the decision to submit the article for publication.
Footnotes
COI and Ethical Adherence The authors report that there are no conflicts of interest for this work. All procedures within this study were conducted with careful Adherence to Ethical Standards, and in full. Compliance With Ethical Standards at the participating universities.
References
- 1.Tolman DL, McClelland SI. Normative sexuality development in adolescence: a decade in review, 2000–2009. J Res Adolesc 2011; 21(1): 242–255. [Google Scholar]
- 2.Center for Disease Control and Prevention (CDC). Youth Risk Behavior Surveillance Survey. Morb Mortal Wkly Rep 2012; 61(4): 1–168. [Google Scholar]
- 3.Center for Disease Control and Prevention (CDC). Youth Risk Behavior Surveillance Survey. Morb Mortal Wkly Rep 2016. https://www.cdc.gov/healthyyouth/data/yrbs/pdf/2015/ss6506_updated.pdf.
- 4.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 2016; 374(9): 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan AD, Schmiege SJ, Broaddus MR. HIV risk reduction among detained adolescents: a randomized, controlled trial. Pediatrics 2009; 124(6): e1180–e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmiege SJ, Broaddus MR, Levin M, Bryan AD. Randomized trial of group interventions to reduce HIV/STD risk and change theoretical mediators among detained adolescents. J Consult Clin Psychol 2009; 77(1): 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Office of AIDS Research (OAR). FY 2017 trans-NIH plan for HIV-related research https://www.oar.nih.gov/strategic_plan/fy2017/OARStrategicPlan2017.pdf.
- 8.Feldstein Ewing SW, Tapert SF, Molina BS. Uniting adolescent neuroimaging and treatment research: recommendations in pursuit of improved integration. Neurosci Biobehav Rev 2016; 62: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills KL, Goddings AL, Herting MM, et al. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage 2016; 141: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2(10):861–863. [DOI] [PubMed] [Google Scholar]
- 11.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 2008; 28(14): 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci 2003;6(3):309–315. [DOI] [PubMed] [Google Scholar]
- 13.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 2004; 24(38): 8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson EE, Jarcho JM, Guyer AE. Social re-orientation and brain development: an expanded and updated view. Dev Cogn Neurosci 2016; 17: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev 2008; 28(1): 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brumback TY, Worley M, Nguyen-Louie TT, Squeglia LM, Jacobus J, Tapert SF. Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev Psychopathol 2016; 28(4pt1): 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldstein Ewing SW, Houck JM, Bryan AD. Neural activation during response inhibition is associated with adolescents’ frequency of risky sex and substance use. Addict Behav 2015; 44: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thayer RE, Montanaro E, Weiland BJ, Callahan TJ, Bryan AD. Exploring the relationship of functional network connectivity to latent trajectories of alcohol use and risky sex. Curr HIV Res 2014; 12(4): 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldstein Ewing SW, Ryman SR, Gillman A, et al. Developmental cognitive neuroscience of adolescent sexual risk and alcohol use. AIDS Behav 2016; Supp 1: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Victor EC, Sansosti AA, Bowman HC, Hariri AR. Differential patterns of amygdala and ventral striatum activation predict gender-specific changes in sexual risk behavior. J Neurosci 2015; 35(23): 8896–8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage 2014; 91: 70–76. [DOI] [PubMed] [Google Scholar]
- 22.Vrtička P, Sander D, Anderson B, Badoud D, Eliez S, Debbané M. Social feedback processing from early to late adolescence: influence of sex, age, and attachment style. Brain Behav 2014; 4(5): 703–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensel DJ, Hummer TA, Acrurio LR, James TW, Fortenberry JD. Feasibility of functional neuroimaging to understand adolescent women’s sexual decision making. J Adolesc Health 2015; 56(4): 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downs JS, Bruine de Bruin W, Fischhoff B, Murray PJ. Behavioral decision research intervention reduces risky sexual behavior. Curr HIV Res 2015; 13(5): 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sales JM, DiClemente RJ, Davis TP, Sullivan S. Exploring why young African American women do not change condom-use behavior following participation in an STI/HIV prevention intervention. Health Educ Res 2012; 27(6): 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koss WA, Frick KM. Sex differences in hippocampal function. J Neurosci Res 2017;95(1–2): 539–562. [DOI] [PubMed] [Google Scholar]
- 27.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 1996; 366(2): 223–230. [DOI] [PubMed] [Google Scholar]
- 28.Sowell ER, Jernigan TL. Further MRI evidence of late brain maturation: limbic volume increases and changing asymmetries during childhood and adolescence. Dev Neuropsych 1998; 14(4): 599–617. [Google Scholar]
- 29.McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res 2011; 224(1): 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res 2008; 32(3): 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol 2009; 14(4): 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol 2015; 66: 295–319. [DOI] [PubMed] [Google Scholar]
- 33.Dorn LD. Measuring puberty. J Adolesc Health 2006; 39(5): 625–626. [DOI] [PubMed] [Google Scholar]
- 34.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44(235): 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45(239): 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage 2014; 88: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp 2014; 35(11): 5633–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baams L, Dubas JS, Overbeek G, van Aken MA. Transitions in body and behavior: a meta-analytic study on the relationship between pubertal development and adolescent sexual behavior. J Adolesc Health 2015; 56(6): 586–598. [DOI] [PubMed] [Google Scholar]
- 39.Smith AR, Chein J, Steinberg L. Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Horm Behav 2013; 64(2): 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinberg L A dual systems model of adolescent risk-taking. Dev Psychobiol 2010; 52(3): 216–224. [DOI] [PubMed] [Google Scholar]
- 41.Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci 2015;16:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci 2015; 16: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnan RE, Callahan TJ, Ladd BO, et al. Evaluating an integrative theoretical framework for HIV sexual risk among juvenile justice involved adolescents. J AIDS Clin Res 2013; 4: 217–236. [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988; 17(2): 117–133. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33(3): 341–355. [DOI] [PubMed] [Google Scholar]
- 46.R Development Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 47.Ludecke D sjmisc: miscellaneous data management tools. R package version 1.4 2016.
- 48.Mirisola A, Seta L. pequod: moderated regression package. R package version 0.0–4 2015.
- 49.Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci 2014; 36(3–4): 147–160. [DOI] [PubMed] [Google Scholar]
- 50.Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 2014; 39: 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price JS, Strong J, Eliassen J, et al. Serotonin transporter gene moderates associations between mood, memory and hippocampal volume. Behav Brain Res 2013; 242: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherf KS, Smyth JM, Delgado MR. The amygdala: an agent of change in adolescent neural networks. Horm Behav 2013; 64(2): 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 54.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 2004; 14(2): 198–202. [DOI] [PubMed] [Google Scholar]
- 55.Cservenka A, Gillespie AJ, Michael PG, Nagel BJ. Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. J Stud Alcohol Drugs 2015; 76(1): 47–56. [PMC free article] [PubMed] [Google Scholar]
- 56.Gilman JM, Kuster JK, Lee S, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci 2014; 34(16): 5529–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse 2013; 39(6): 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawamichi H, Sasaki AT, Matsunaga M, et al. Medial prefrontal cortex activation is commonly invoked by reputation of self and romantic partners. PLoS One 2013; 8(9): e74958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kühn S, Gallinat J. Brain structure and functional connectivity associated with pornography consumption: the brain on porn. JAMA Psychiatry 2014; 71(7): 827–834. [DOI] [PubMed] [Google Scholar]
- 60.Geier CF. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm Behav 2013; 64(2): 333–342. [DOI] [PubMed] [Google Scholar]
- 61.Donovan JE, Jessor R, Costa FM. Syndrome of problem behavior in adolescence: a replication. J Consult Clin Psychol 1988; 56(5): 762–765. [DOI] [PubMed] [Google Scholar]
- 62.Feldstein Ewing SW, Bryan AD. A question of love and trust? The role of relationship factors in adolescent sexual decision-making. J Dev Behav Pediatr 2015; 36(8): 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broaddus MR, Schmiege SJ, Bryan AD. An expanded model of the temporal stability of condom use intentions: gender-specific predictors among high-risk adolescents. Ann Behav Med 2011; 42(1): 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoppe MJ, Graham L, Wilsdon A, Wells EA, Nahom D, Morrison DM. Teens speak out about HIV/AIDS: focus group discussions about risk and decision-making. J Adolesc Health 2004; 35(4): 345.e27–345.e35. [PubMed] [Google Scholar]
- 65.Ott MA, Millstein SG, Ofner S, Halpern-Felsher BL. Greater expectations: adolescents’ positive motivations for sex. Perspect Sex Reprod Health 2006; 38(2): 84–89. [DOI] [PubMed] [Google Scholar]
- 66.Stanton B, Li X, Black M, et al. Sexual practices and intentions among preadolescent and early adolescent low-income urban African-Americans. Pediatrics 1994; 93(6 Pt 1): 966–973. [PubMed] [Google Scholar]
- 67.Bryan AD, Aiken LS, West SG. Young women’s condom use: the influence of acceptance of sexuality, control over the sexual encounter, and perceived susceptibility to common STDs. Health Psychol 1997; 16(5): 468–479. [DOI] [PubMed] [Google Scholar]
- 68.Gebhardt WA, Kuyper L, Greunsven G. Need for intimacy in relationships and motives for sex as determinants of adolescent condom use. J Adolesc Health 2003; 33(3): 154–164. [DOI] [PubMed] [Google Scholar]
- 69.Widdice LE, Cornell JL, Liang W, Halpern-Felsher BL. Having sex and condom use: potential risks and benefits reported by young, sexually inexperienced adolescents. J Adolesc Health 2006; 39(4): 588–595. [DOI] [PubMed] [Google Scholar]
- 70.Pulerwitz J, Amaro H, De Jong W, Gortmaker SL, Rudd R. Relationship power, condom use and HIV risk among women in the USA. AIDS Care 2002; 14(6): 789–800. [DOI] [PubMed] [Google Scholar]
- 71.Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport 2001; 12(2): 427–433. [DOI] [PubMed] [Google Scholar]
- 72.Hockenberry S, Puzzanchera C. Juvenile Court Statistics 2013 Pittsburgh, PA: National Center for Juvenile Justice; 2015. [Google Scholar]
- 73.Skeem JL, Scott E, Mulvey EP. Justice policy reform for high-risk juveniles: using science to achieve large-scale crime reduction. Annu Rev Clin Psychol 2014;10:709–739. [DOI] [PubMed] [Google Scholar]
- 74.Feldstein SW, Ginsburg JID. Motivational interviewing with dually diagnosed adolescents in juvenile justice settings: A review. Brief Treat Crisis Intervent 2006; 6(3): 218–233. [Google Scholar]
- 75.Salvador JG, DeVargas EC, Feldstein Ewing SW. Who are Hispanic youth? Consideration for adolescent addiction clinical research and treatment. Alcoholism Treat Quarterly 2015; 33(3): 348–362. [Google Scholar]
- 76.CNN.com. White children in the minority in 10 states – This Just In – CNN.com Blogs. (Vol. 2013). news.blogs.cnn.com, 2011. [Google Scholar]
- 77.Chappell B. For US children, minorities will be the majority by 2020, census says. NPR. 2015 https://www.npr.org/sections/thet-wo-way/2015/03/04/390672196/for-u-s-children-minorities-will-be-the-majority-by-2020-census-says.NPR. Accessibility verified October 27, 2016.
- 78.HHS. The changing face of America’s adolescents https://www.hhs.gov/ash/oah/facts-and-stats/changing-face-of-americas-adolescents/index.html. 2016. Accessibility verified October 27, 2016.
- 79.Feldstein Ewing SW, Montanaro EA, Gaume J, Caetano R, Bryan AD. Measurement invariance of alcohol instruments with Hispanic youth. Addict Behav 2015; 46: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldstein Ewing SW, Wray AM, Mead HK, Adams SK. Two approaches to tailoring treatment for cultural minority adolescents. J Subst Abuse Treat 2012; 43(2): 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feldstein Ewing SW, Venner KL, Mead HM, Bryan AD. Exploring racial/ethnic differences in substance use: a preliminary theory-based investigation with juvenile justice-involved youth. BMC Pediatr 2011; 11: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry 2010; 49(6): 552–560. [DOI] [PubMed] [Google Scholar]
- 83.Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2008; 47(11): 1289–1298. [DOI] [PubMed] [Google Scholar]
- 84.Feldstein Ewing SW, Magnan RE, Houck CD, Morgan M, Bryan AD. Associations between CDH13 variants and the initiation of alcohol use and sexual intercourse with high-risk Hispanic and Caucasian youth. Online J Rural Urban Res 2014; 4(1): 1–18. [Google Scholar]
- 85.Mustanski B Future directions in research on sexual minority adolescent mental, behavioral, and sexual health. J Clin Child Adolesc Psychol 2015;44(1):204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ansara YG. Improving research methodology in adolescent sexual health research. J Adolesc Health 2015;56(4):367–369. [DOI] [PubMed] [Google Scholar]
- 87.Stahlman S, Lyons C, Sullivan P, et al. HIV incidence among gay men and other men who have sex with men in 2020: where is the epidemic heading? Sex Health 2016; 14(1): 5–17. [DOI] [PubMed] [Google Scholar]