Fig. 1.
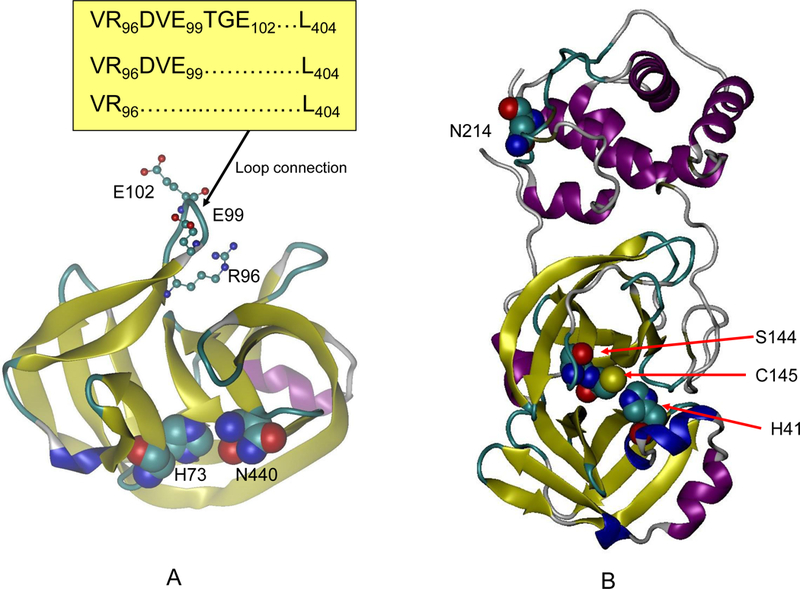
Allosteric proteins are sensitive to crowding effects. (A) The mini intein activity can be allosterically modulated by a loop distant from the active site. Two residues in the active site (H73 and N440) are represented by large balls; and residues in the loop are represented by small balls and sticks. Three loops with different sizes were tested in reference [35]. Active intein has a longer loop (VR96DVE99TGE102L404). While reducing the loop length to VR96DVE99... L404 still preserved the activity, further shortening to VR96L404 inactivates the enzyme. (B) The structure of allosteric protein SARS-Co 3CL peptidase, for which the catalytic activity increases in a crowded environment. Three active site residues (H41, S144, and C145) are shown as large balls in one domain. The mutant position N214 is shown as large balls in another domain.
