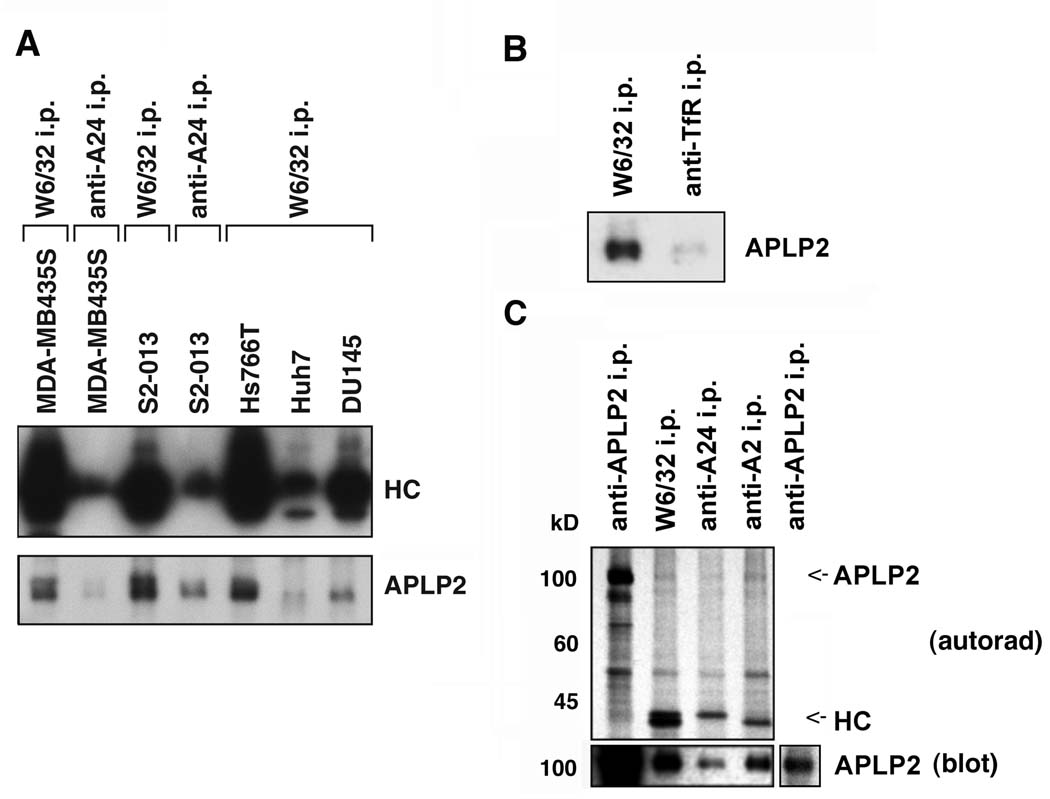
Figure 5.
APLP2 co-precipitated with HLA class I molecules in lysates of human tumor cell lines, more strongly with HLA-A2 than with HLA-A24. (A) Immunoprecipitations were performed on lysates of the indicated cell types with W6/32 or an antibody recognizing HLA-A24, and the immunoprecipitates were electrophoresed on 4→20% acrylamide Tris-glycine gels, transferred to a Western blot, and probed with HC10 for the immunoprecipitated HLA class I heavy chains (HC), or with an antiserum recognizing APLP2. (B) HLA class I molecules and transferrin receptors (as a negative control) were immunoprecipitated from lysates of S2-013 cells with W6/32 and anti-transferrin receptor serum, respectively. The immunoprecipitates were electrophoresed on a 4→20% acrylamide Tris-glycine gel, blotted, and probed with anti-APLP2 serum. (C) Immunoprecipitations were done on lysates of 35S-methionine/cysteine-labeled pancreatic tumor S2-013 cells with an antiserum recognizing APLP2, the W6/32 antibody, an antibody recognizing HLA-A24, or an antibody recognizing HLA-A2. The samples were electrophoresed on 4→20% acrylamide Tris-glycine gels. For autoradiography, the proteins were transferred from the gel to a membrane that was dried and exposed to Kodak BioMax film (upper panel, labeled as autorad), and also transferred to a second membrane that was probed with an antiserum recognizing APLP2 (bottom panel, labeled as blot). The APLP2 and heavy chain (HC) bands are indicated on the autoradiograph with arrows (the HLA-A24 and –A2 bands are of slightly different molecular weights). The additional section on the far right of the bottom panel shows a lighter exposure of the APLP2 blot so that the APLP2 band can be seen more clearly.