Figure 3. Expression of CD4+ T cell-, but not CD8+ or B cell-module-associated genes at 26 weeks predicts C-peptide loss in rituximab-treated patients.
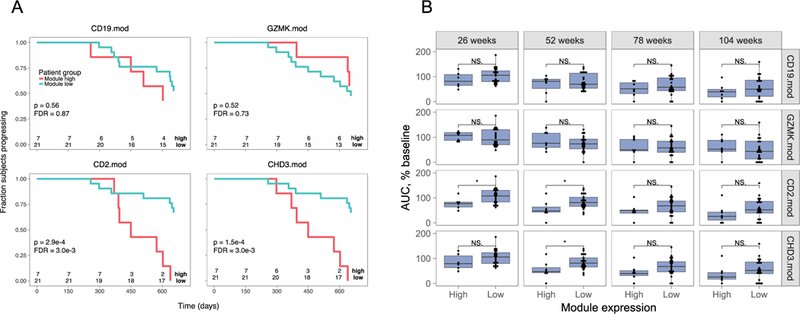
A) Rituximab-treated subjects were split into module high (top 25%) and module low (bottom 75%) groups based on log2 median module gene counts+1 values from RNA-seq profiles collected at week 26. Shown are Kaplan-Meier plots for progression, measured as time to 50% of baseline C-peptide, versus time. Survdiff (43) p-values were calculated and adjusted for multiple testing (40). Rituximab-treated subjects were stratified by median gene expression in B cell module, CD19.mod; CD8+ T cell-associated module, GZMK.mod; and CD4+ T cell-associated modules CD2.mod and CHD3.mod. Numbers at bottoms of panels are numbers of subjects at risk. Numbers of subjects tested are indicated at the bottom of each panel. B) Expression of CD4+ T cell-, but not CD8+ or B cell-module-associated genes at 26 weeks predicts C-peptide AUC levels at 52 weeks in rituximab-treated subjects. Shown are C-peptide AUC levels (% of baseline), across all visits of rituximab-treated subjects. Subjects were stratified into module high and nodule low subsets based on the expression of the indicated module gene expression at week 26, as described in Figure 3. Asterisks indicate level of significance of Wilcox test p-values: *, p-value<0.05 and p-value >=0.01. Numbers of subjects tested were the same as in panel A.
