Abstract
Importance:
Developmental origins of mental illness are incompletely understood. Although development of autism and schizophrenia are linked to infections during fetal life, whether more common psychiatric conditions like depression might begin in utero is unknown.
Objective:
To estimate the psychopathology risk imparted from fetal exposure to any hospitalized maternal infection during pregnancy.
Design:
Directed Acyclic Graphs (DAGs) were developed from a systematic literature review to determine Cox Regression models for psychopathology risk in the children. Results were evaluated using Probabilistic and Simple Bias Analyses.
Setting:
All Swedish hospitals.
Participants:
Swedish children born between 1973–2014 were followed for up to 41 years using linked population-based registries. Children were excluded if born too late to contribute person-time, died before being at risk for the outcome or were missing particular model data. Infection and psychiatric diagnoses were derived using codes from hospitalizations.
Exposures:
Hospitalization during pregnancy with (1) any maternal infection, (2) severe maternal infection and (3) urinary tract infection.
Main Outcomes:
Inpatient diagnosis of autism, depression, bipolar disorder, or psychosis among offspring.
Results:
We followed 1,791,520 Swedish-born children (49% female) from birth up to 41 years with 32,125,813 person-years. Within our DAG framework of assumptions, fetal exposure to any maternal infection increased risk of an inpatient diagnosis with autism or depression in the child [autism hazard ratio (HR)=1.79, 95% confidence interval (CI): 1.34–2.4; depression HR=1.24, 95%CI:1.08–1.42]. Both severe infections and urinary tract infections increased risk for autism and depression and were robust to moderate unknown confounding. The depression relationship was vulnerable to bias from loss to follow-up, but separate data from the Swedish Death Registry demonstrated increased risk of suicide among individuals exposed to pregnancy infection. We found no evidence for increased risk of bipolar disorder or psychosis among children exposed to infection in utero within our models.
Conclusions and Relevance:
Our findings suggest that fetal exposure to a maternal infection with hospitalization increased risk for autism and depression, but not bipolar or psychosis, during the child’s life. These results emphasize the importance of avoiding infections during pregnancy, which may impart subtle fetal brain injuries contributing to development of autism and depression.
Background
A large body of evidence indicates that particular infections in pregnancy lead to fetal brain injury, neurodevelopmental abnormalities and an elevated lifelong risk for certain psychiatric disorders in the children.1–4 Early epidemiological evidence for a relationship between infection in pregnancy and psychopathology was evident in Scandinavian studies of schizophrenia risk following influenza exposure in utero3 and subsequent studies have demonstrated similar associations with other viral (measles, rubella, varicella-zoster, polio, herpes), bacterial and parasitic infections during pregnancy.5–7 Other studies found associations between maternal infection and development of bipolar disorder and autism.8–10 While compelling, these studies have mainly focused on linking specific infections with a particular psychopathology, with few exceptions11, rather than determining a generalized effect of infection and inflammation during pregnancy on a broad spectrum of psychopathology.
Whether maternal infection and inflammation can alter fetal neurodevelopment to a degree that imparts risk for a broad spectrum of psychopathology across the child’s lifetime is unknown. Several mechanisms of neuronal injury have been postulated to occur in the developing brain as a result of inflammation and glial production due to activation of mast cells, microglia and astrocytes.7–18 Maternal and fetal inflammatory responses to infection may alter fetal neurodevelopment as suggested in some children with autism, who display a chronic state of inflammation in the periphery as well as in brain.10,24–26 Further, changes in placental serotonin production due to maternal inflammation have been suggested to lead to aberrant neurodevelopment.27 Finally, the idea that some psychiatric disorders have a common mechanism of pathogenesis is supported by recent evidence implicating shared molecular pathways of transcriptional dysregulation and a common polygenic origin for autism, schizophrenia and bipolar disorder28,29.
We hypothesized that fetal exposure to maternal infection or associated inflammation increases the future risk for the child of a broad scope of psychopathology such as autism spectrum disorder, bipolar disorder, depression and psychosis including schizophrenia. We further hypothesized that the magnitude of risk for psychopathology in the child differs by the type and severity of the maternal infection.
Methods
Study Design
We obtained data on all births between 1973–2014 in the Swedish population-based birth registry, which was linked to hospital inpatient, demographic, education and death registries by each person’s unique identification number30. Raw data included some births in 1972 and 2015 that were excluded. Statistics Sweden de-identified the data to maintain confidentiality; therefore, informed consent was not required. Ethical approval was obtained to link birth and registry data from the regional ethical review board of the University of Gothenburg (Gothenburg, Sweden; 437–15) and reciprocally at Seattle Children’s Hospital (Seattle, WA, USA; STUDY00000634).
Exposure
Fetal exposure to maternal infection was defined using Swedish ICD (International Classification of Diseases versions 8, 9 and 10) hospitalization codes. Three pre-specified exposure categories were used in pregnant women hospitalized with a diagnosis of: (1) any maternal infection (eTable1), (2) severe infections limited to sepsis, meningitis/encephalitis, pneumonia, influenza, pyelonephritis, chorioamnionitis (eTable2), and/or (3) urinary tract infection (eTable2). We used both primary and secondary infection diagnosis codes that occurred during any pregnancy hospitalization except the delivery admission, because we could not determine that infection preceded the birth for this admission. There was one exception to this rule; we used a chorioamnionitis diagnosis from the delivery admission, because this could have only occurred before delivery.
Primary Outcomes
Primary study outcomes were (1) autism, (2) bipolar disorder, (3) depression, and (4) psychosis (including schizophrenia), which were defined by Swedish ICD8, 9 and 10 codes during an inpatient hospitalization (eTable3).
Inclusion and Exclusion Criteria
We included all children born in Sweden from 1973 to 2014 for our models with depression, bipolar disorder and psychosis (Fig. 1). In the autism model, we included all children born in Sweden from 1987 to 2014, because 1987 coincided with the introduction of autism as a new diagnosis and implementation of ICD9 coding. As psychopathology diagnoses are unusual prior to certain ages, children were only considered at risk for autism after age 2, depression after age 6, and psychosis and bipolar disorder after age 10.
Figure 1.
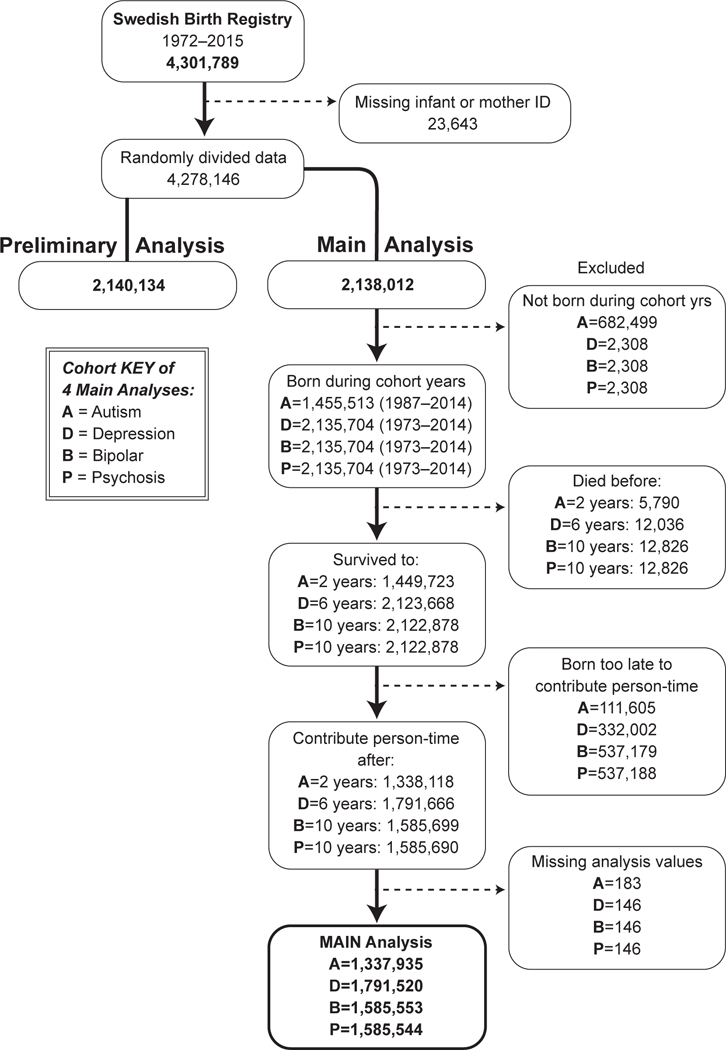
Inclusions and exclusions by psychopathology outcome. This diagram shows cohort numbers used to analyze fetal exposure to infection and each psychopathology type, which varied due to differing inclusion and exclusion criteria.
Statistical Analysis
We reviewed 12,264 abstracts and articles to develop a series of directed acyclic graphs (DAG) in order to characterize the relationships among maternal infection during pregnancy, diagnosis of psychopathology in the child and other important variables. These models were based on the best available evidence and subject area expertise when evidence was not available. The authors reviewed and approved the final DAG models (Supplemental Methods, eFigures 1-12).
On the basis of these DAGs, we used established analytic rules with Dagitty web application and the R package to determine whether a given variable should be considered as a confounder, collider or neither a confounder or collider. These tools were also used to establish the minimally sufficient adjustment sets of variables for regression to estimate the total effects of exposure to infection during fetal life on subsequent development of psychopathologies in childhood and adulthood.31 If there was disagreement about a variable’s status, we ran the analysis with and without the variable to determine how the effect estimate changed (e.g. maternal mental health, eTable 4). Statistically equivalent DAG models were also evaluated for alternate frameworks.
According to best practice and consistent with study power, we split the data in half; one-half was used for preliminary power analyses to determine whether there was sufficient power to examine infections by trimester as well as asymptomatic bacteriuria and vaginal tract infections as separate categories. The second half of the data was used for 8 pre-specified analyses with Bonferroni correction (type I error rate of 0.05 and after correction 0.0063). In the first four analyses, we investigated the relationship between maternal infection and 1) bipolar disorder, 2) psychosis including schizophrenia, 3) autism, or 4) depression. The final four analyses studied the effect of a severe maternal infection on diagnosis of 5) autism and 6) depression or the effect of a maternal urinary tract infection (UTI) on diagnosis of 7) autism or 8) depression. Based on the DAG models, we used Cox Proportional Hazards regression to generate Hazard Ratios (HR). We ensured that the proportional hazards assumption was met using individual and global chi-squared tests and examination of Schoenfeld residual plots. In the majority of models, delivery year and maternal tobacco use violated the proportional hazards assumption; therefore, we estimated HR with maternal tobacco use and 10-year birth epoch as strata. We also adjusted for maternal age, maternal asthma, maternal diabetes and premature rupture of membranes. Other variables examined are shown in eFigures 1-12. To account for non-independence of siblings, we used robust sandwich variance estimators. Unadjusted cumulative hazards were also plotted for each exposure and outcome.
Bias analyses
We used two methods of bias analysis to interrogate our results: 1) Probabilistic Bias Analysis to assess for an unknown confounder and 2) Simple Bias Analysis to assess for outcome misclassification (differential loss to follow-up) and exposure misclassification (inaccurate infection coding).32
Results
Study Population and Cumulative Hazard Curves
We analyzed linked Swedish pregnancy and birth records for 4,278,155 neonates, as well as subsequent hospitalization records for the children up to 41 years after birth. We used half the data for preliminary power analyses and the second half (2,138,012 records) for the main analysis (Fig. 1). Study population characteristics are reported in Table 1.
Table 1.
Maternal, Delivery and Infant Characteristics by Infection Status
| No Infection (n= 2,108,156) |
Any Infection (n= 29,856) |
|
|---|---|---|
| Maternal characteristics | ||
| Maternal age - mean (sd) | 28.7 (5.3) | 27.6 (5.6) |
| Maternal tobacco use** - n (%) | 324,493 (15.4) | 5,671 (19) |
| Maternal asthma - n (%) | 4,844 (0.2) | 158 (0.5) |
| Maternal seizures - n (%) | 3,592 (0.2) | 106 (0.4) |
| Maternal hypertension - n (%) | 53,314 (2.5) | 1,007 (3.4) |
| Maternal diabetes mellitus - n (%) | 6,358 (0.3) | 291 (1.0) |
| Maternal mental health diagnosis - n (%) | 15,820 (0.75) | 596 (2.0) |
| Delivery characteristics | ||
| Gestational age - mean (sd) | 39.4 (1.9) | 39 (2.4) |
| Prolonged labor - n (%) | 27,456 (1.3) | 493 (1.7) |
| Preterm premature rupture of membranes - n (%) | 3,216 (0.2) | 138 (0.5) |
| Antepartum hemorrhage - n (%) | 35,748 (1.7) | 1,045 (3.5) |
| Infant characteristics | ||
| Birthweight in grams – mean (sd) | 3,508 (579) | 3,415 (643) |
| Female sex - n (%) | 1,024,770 (48.6) | 14,474 (48.5) |
| Small for gestational age* - n (%) | 66,785 (3.2) | 1,201 (4.0) |
| Large for gestational age* - n (%) | 67,774 (3.2) | 1,057 (3.5) |
| Bronchopulmonary dysplasia or Respiratory Distress Syndrome - n (%) | 5,666 (0.3) | 171 (0.6) |
| Intraventricular hemorrhage - n (%) | 5,666 (0.3) | 171 (0.6) |
| Hyperbilirubinemia - n (%) | 69,750 (3.3) | 1,495 (5.0) |
| Fetal alcohol syndrome - n (%) | 6,679 (0.3) | 195 (0.7) |
| Outcomes | ||
| Bipolar disorder - n (%) | 4,402 (0.2) | 71 (0.2) |
| Psychosis, including schizophrenia - n (%) | 4,307 (0.2) | 75 (0.3) |
| Autism - n (%) | 5,003 (0.2) | 133 (0.5) |
| Depression - n (%) | 20,749 (1.0) | 409 (1.4) |
The data show number (%) of pregnant women or infants by exposure status or mean (standard deviation) for maternal age, gestational age, and birthweight.
Numbers to indicate neonates that were either large or small for gestational age were estimated using the Marsal method.49
Tobacco status is not known for women who gave birth before 1982.
To evaluate the psychopathology risk in the child after fetal exposure to any hospitalized maternal infection, unadjusted cumulative hazard curves were generated by infection status for each outcome (Fig. 2). The risk for hospital admission with psychosis and bipolar disorder appeared similar between children exposed and not exposed to any maternal infection with hospitalization in utero (Fig. 2A-B). However, the cumulative hazard for admission with autism or depression in children exposed to infection in utero was significantly greater by ages 7 and 21 years, respectively, than for children not exposed to infection (Fig. 2C-D). The number of outcomes, person-years and unadjusted rate ratios for any maternal infection and risk for a neuropsychiatric disorder are presented in eTables 5-8.
Figure 2.
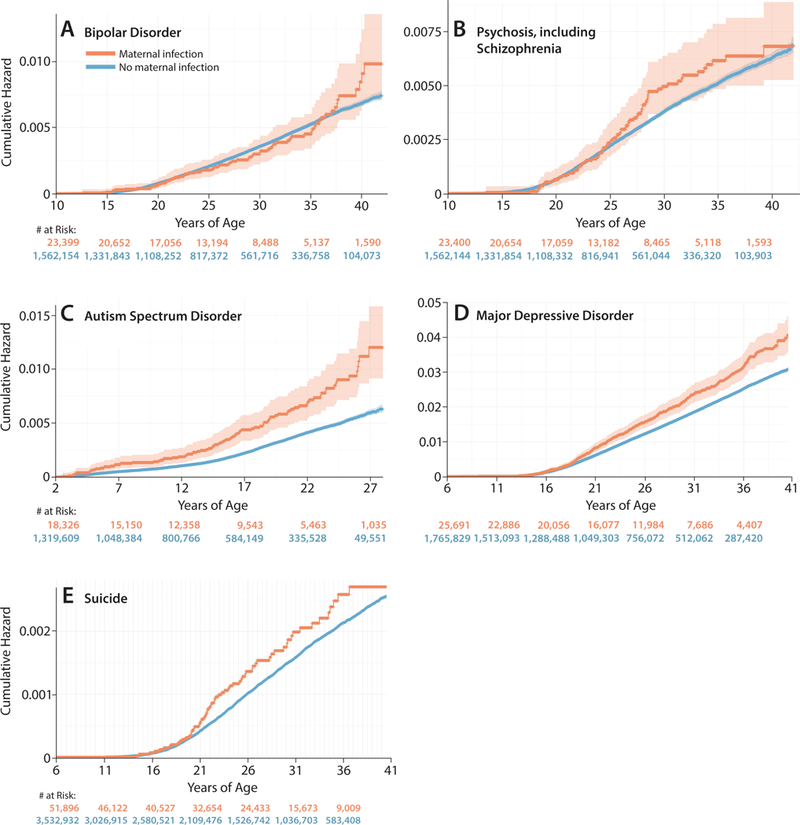
Lifetime risk for psychopathology in the child after fetal exposure to maternal infection. Unadjusted cumulative hazard curves demonstrate the risk for autism (A), depression (B), bipolar disorder (C), psychosis (D) and death by suicide (E) among individuals exposed and not exposed to infection in utero. The orange and blue lines indicate children and adults exposed or unexposed to infection, respectively, during fetal life. Shading around the lines indicates the 95% confidence interval.
To determine if the type of maternal infection changed the risk of autism or depression, we modeled the psychopathology risk imparted by a composite of severe maternal infections (sepsis, pneumonia, pyelonephritis, meningitis/encephalitis, influenza, chorioamnionitis) versus UTI, which may represent a more limited infection. In unadjusted cumulative hazard curves, children or adults exposed to severe maternal infections during fetal life had similar magnitudes of increased risk for autism and depression compared to children exposed to a maternal UTI (Fig. 3). The number of outcomes, person-years and unadjusted rate ratios for severe maternal infections or UTI and risk for a neuropsychiatric disorder are presented in eTables 9-12.
Figure 3.
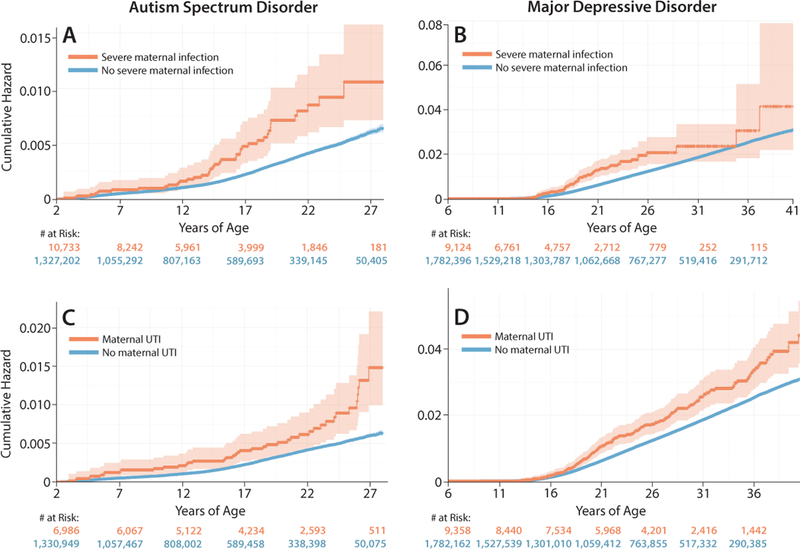
Lifetime risk for autism or depression in the child after fetal exposure to maternal infection by type of infectious exposure. Unadjusted cumulative hazard curves demonstrate risk for autism across the child’s lifetime by fetal exposure to either (A) severe maternal infection (sepsis, meningitis/encephalitis, pneumonia, influenza, pyelonephritis, chorioamnionitis) or (C) urinary tract infection. Similarly, the risk for depression in the child across their lifetime following fetal exposure to either (B) severe maternal infection or (D) urinary tract infection is illustrated by the cumulative hazard function. The orange and blue lines indicate children and adults exposed or unexposed, respectively, to a maternal severe infection or urinary tract infection during fetal life. Shading around the lines indicates the 95% confidence interval.
Cox Regression Cumulative Hazards
DAG models informed a minimally sufficient adjustment set for estimating the effect of pregnancy infections on future psychopathology risk in the child, which included 10-year epoch of delivery and specific maternal exposure variables (age, tobacco use, asthma, diabetes and premature rupture of membranes). In Cox regression models, there was a 79% increased risk of an autism diagnosis and 24% increased risk of a depression diagnosis among children and adults exposed to any maternal infection during pregnancy (Table 2; autism HR=1.79, 95%CI:1.34–2.4; depression HR=1.24, 95%CI:1.08–1.42; eFigures 7-12). There was no apparent increased risk of bipolar disorder (HR=0.99, 95%CI: 0.71–1.38) or psychosis diagnoses (HR=1.14, 95%CI:0.83–1.57), including schizophrenia, in childhood or adulthood following fetal exposure to maternal infection (eFigures 1-6). Additional analyses with maternal mental health as a confounder did not yield significantly different effect estimates (eTable 4). When we analyzed risk for psychopathology by infection type, we found similar magnitudes of increased autism and depression risk regardless of whether the exposure was a severe maternal infection or UTI (Table 2). For example, the hazard ratio for hospital admission with an autism diagnosis in childhood or adulthood after exposure to infection during fetal life was 1.85 (95%CI:1.18–2.78) for a severe infection and 1.89 (95%CI:1.23–2.9) for UTI.
Table 2.
Hazard ratios for development of psychopathology by infection type in pregnancy
| Infection Type | Psychopathology Outcome | Adjusted Hazard Ratio | 95% CI* | |
|---|---|---|---|---|
| Any Maternal Infection | Autism | 1.79 | 1.34 | 2.4 |
| Depression | 1.24 | 1.08 | 1.42 | |
| Psychosis | 1.14 | 0.83 | 1.57 | |
| Bipolar† | 0.99 | 0.71 | 1.38 | |
| Severe Maternal Infection | Autism | 1.81 | 1.18 | 2.78 |
| Depression | 1.24 | 0.88 | 1.73 | |
| Maternal Urinary Tract Infection | Autism | 1.89 | 1.23 | 2.9 |
| Depression | 1.3 | 1.04 | 1.61 | |
All models included maternal age, maternal asthma, maternal diabetes mellitus, premature rupture of membranes, maternal tobacco status and robust sandwich variance estimators for lack of independence among sibling births.
For all models, stratification was used for 10-year epoch of birth and maternal tobacco status to avoid violating the proportional hazard assumption.
Bonferroni correction was used to adjust confidence intervals for 8 pre-specified comparisons shown in the table.
Stratification was additionally used for premature rupture of membranes and maternal age because of proportional hazards assumption violations.
Bias Analyses
We used two methods of bias analysis to evaluate result sensitivities to introduction of an unknown confounder and loss to follow-up bias. First, we used Probabilistic Bias Analysis to introduce a hypothetical moderate confounder to determine sensitivity of the results (eFigure 14).32 However, measures of effect remained significant and only slightly decreased for all statistically significant results for development of autism or depression.
Second, we used Simple Bias Analysis32 to determine the effect of misclassification of outcome bias from loss to follow-up, which would occur when a child was diagnosed with psychopathology (i.e. autism) but never admitted to the hospital during the study period. Notably, inpatient admission was required in our study to capture psychopathology diagnoses through the Swedish Inpatient Health Registry. Our findings suggest that a population prevalence of autism spectrum disorder between 2–3% would be required to negate these results based on a misclassification of outcome bias (eTable 13). Although estimates of autism prevalence have increased over time with highest estimates of prevalence in Stockholm recently published at 2.5%33, prior studies in Sweden encompassing most of the study period estimated autism prevalence between 0.2 and 1%.34
For the outcome of depression, when we assumed a population prevalence of only 5%, there was no increased risk of depression among children and adults exposed to any maternal infection or UTI during fetal life (eTable 14). Depression estimates in Sweden are higher than 5% suggesting that this analysis is vulnerable to a misclassification of outcome bias from loss to follow-up.35 To interrogate these results with external data, we used the Swedish National Death Registry to examine cumulative hazard curves for suicide among individuals exposed during fetal life to a hospitalized maternal infection. As the National Death Registry is inclusive of the entire Swedish population, it is not vulnerable to bias from loss to follow-up like the inpatient registry is. Interestingly, the cumulative hazard for death by suicide among adults exposed to infection during fetal life was significantly greater compared to unexposed individuals starting at age 21, which mirrored the results from the inpatient registry for depression (Fig. 2E). Although the Cox regression models for depression using inpatient data were vulnerable to a misclassification of outcome bias, descriptive suicide data supported the results and were not subject to the same bias.
Lastly, we did a series of analyses to examine whether our results were sensitive to a misclassification of exposure bias, which may occur when maternal infection is misdiagnosed or miscoded during pregnancy. We found that the risk ratios corrected for misdiagnosis or miscoding were larger than the original effect estimates for maternal infection on autism and depression, severe maternal infection (autism only) or maternal UTI (eTables 15-19). This suggests that our results are robust to possible bias from misclassification of exposure.
Discussion
The effect of infection during pregnancy on the fetal brain and risk for subsequent development of neuropsychiatric disorders is understudied. In the Swedish population, we found compelling evidence that fetal exposure to infection (or inflammation) when the mother was hospitalized increased risk for hospital admission with autism during childhood and adulthood. These effects were observed irrespective of whether the exposure was a maternal severe infection (sepsis, influenza, pneumonia, meningitis/encephalitis, chorioamnionitis and pyelonephritis) or UTI during pregnancy. Bias analyses revealed that autism results were robust to adjustment for a moderate unknown confounder, but that the depression results were vulnerable to bias from our inability to capture diagnoses among those never admitted to the hospital after birth (loss to follow-up). However, separate descriptive data from the National Death Registry, which was not subject to loss to follow-up, supported the depression results by demonstrating increased risk of suicide among adults exposed to infection during fetal life. We did not find convincing evidence that maternal infection during gestation increased lifetime risk of bipolar disorder or psychosis, including schizophrenia. Overall, our findings provide evidence for a fetal origin of some portion of autism and depression across a spectrum of maternal infections in pregnancy.
The earliest evidence for a fetal origin of psychiatric disease came from correlations of birth season with incidence of schizophrenia and later through associations with maternal influenza infection.3 Subsequent studies have yielded mixed results36,37 and recent evidence suggests that the relationship with inflammatory exposures in pregnancy may be complicated by genetic susceptibility for both schizophrenia and autism.29,38–40 Unlike prior epidemiologic studies, our work used a literature based variable framework (DAG models) and descriptive cumulative hazard curves to demonstrate that maternal infection during pregnancy increased risk not only for autism, but also possibly for depression. A few studies have investigated lifetime risk of depression for the child after exposure to particular infections, but these have yielded mixed results.41,42 Although vulnerable to possible loss to follow-up bias, our study provides suggestive evidence for a fetal origin for depression, with separate support using suicide data from the Swedish National Death Registry. Although little is known about the scientific basis to link aberrant fetal neurodevelopment with subsequent risk for depression, infection and inflammation in the pregnant mouse lead to alterations in placental serotonin production and dysgenesis of serotonergic neurons in the fetal brain. These new findings suggest an important possible biological basis for a fetal origin for depression and suicide.27
Our results of increased autism risk following fetal exposure to infection are consistent with other epidemiologic and animal studies, which suggest that inflammation during gestation alters brain architecture or transcriptional programs.1,43,44 Interestingly, and aligned with another study from Sweden, we found no evidence that maternal infection increased lifetime risk of psychosis or bipolar disorder.45 However, the descriptive cumulative hazard curve for admission with psychosis suggests that infection may increase risk earlier, but not later in the child’s lifetime. Notably, our results cannot exclude the possibility of increased risk for psychopathology as a result of a dual “hit”: an inflammatory fetal brain injury on a background of genetic susceptibility.
Although we expected that fetal exposure to “severe” infection would increase risk for psychopathology compared to limited infections such as UTI, we did not find a difference in these models. The effect of UTI on uterine and placental inflammation during pregnancy is unknown, but may be sufficient to alter neuropsychiatric risk for the fetus. A few studies of UTI in pregnancy have found increased risk of fetal morbidity and developmental delay.46 While it is possible that the diagnosis of a UTI in hospitalized women may have reflected a more severe infection (e.g. pyelonephritis), our findings suggest that further study is warranted to quantify the inflammatory effects of a UTI in pregnancy on the fetus.
A clear strength of the study was the conservative nature of our analysis and use of mechanistic DAG models to specify a priori the complicated relationships among maternal, paternal, perinatal and psychiatric outcome variables. We also interrogated the sensitivity of our results to potential biases. Not that DAG modeling is limited by the degree to which variables are included and relationships appropriately specified. Although the DAGs did not indicate that adjustment for socioeconomic status was required, further analysis adjusting for occupation, education, or income would have enriched the study, but income variables were incomplete in our dataset. As our study was restricted to Swedish pregnancies, it is possible that findings may not be generalizable to other populations. Lastly, we acknowledge that data related to maternal infection and psychiatric outcomes from birth and health registries were derived only from inpatient hospitalizations. Our results may not translate to infections diagnosed in the outpatient setting. Notably, bias analyses and suicide data from the death registry suggest that our results are somewhat robust to the probable underestimate of psychiatric disorders in our study, which occurred when participants were not admitted to the hospital after birth (“lost to follow-up”).
Overall, we found compelling evidence that exposure to infection during fetal life increased risk of autism and possibly depression in the child within our DAG model assumptions. While the individual risk appears small, the population effects are potentially large. Our findings amplify the urgency to better understand the role of infection during pregnancy on fetal brain development and suggest that infection prevention (e.g. influenza vaccination) or anti-inflammatory therapies47,48 may be important strategies for the primary prevention of some portion of autism and depression.
Supplementary Material
Key Points.
Question:
Does exposure to maternal infection during pregnancy increase the long-term risk for major psychiatric disorders in the child?
Findings:
In this Swedish population-based cohort study of children born between 1973–2014, we found that exposure to infection in pregnancy significantly increased risk for autism spectrum disorder (HR=1.79) and depression (HR=1.24) within our framework assumptions.
Meaning:
Maternal infection during pregnancy may be responsible for some portion of autism and depression in childhood and adulthood among the exposed offspring.
Acknowledgments
We thank Ylva Folkesson (Gothenberg University), Geena Gallardo (University of Washington) and Sylvia Lago (Seattle Children’s Hospital) for administrative assistance. Peggy Cruse, MLIS (Seattle Children’s Hospital) provided invaluable assistance with the literature review. We thank Jan Hamanishi (University of Washington) for technical assistance with preparation of the figures. We also thank Dr. Ann Vander Stoep, PhD (University of Washington) for expertise and advice related to psychiatric epidemiology.
This work was supported by: Goljes minnesfond stiftelsen Sigurd och Elsa (LA2015–0121, V.S.) and Fru Mary von Sydows, född Wijk, donationsfond (nr3615 to V.S.), the Agreement concerning research and education of doctors (ALFGBG-717501, ALFGBG-426411 to D.M. and B.J.; Sahlgrenska University Hospital, Sahlgrenska Academy, Gothenburg, Sweden), the University of Washington Neonatal Biology Research Fund (B.J.S.a-H and S.C.), T32 GM008244 (B.J.S.a-H), Department of Pediatrics at the University of Washington (B.J.S.a-H.), R01 AI33976 (L.R and K.A.W) and the Department of Obstetrics & Gynecology at the University of Washington (K.A.W). No authors have conflicts of interest. No funding source was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors have no financial or other conflicts of interest.
Dr. Benjamin al-Haddad had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Benjamin al-Haddad (Seattle Children’s Hospital and University of Washington) and Ms. Dominika Modzelewska (Sahlgrenska Academy, University of Gothenburg) conducted and are responsible for the data analysis.
References
- 1.Limperopoulos C, Bassan H, Sullivan NR, et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics 2008;121(4):758–765. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canetta SE, Bao Y, Co MDT, et al. Serological Documentation of Maternal Influenza Exposure and Bipolar Disorder in Adult Offspring. Am J Psychiatry 2014;171(5):557–563. doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 1988;45(2):189–192. [DOI] [PubMed] [Google Scholar]
- 4.Machón R, Mednick S, Huttunen M. Adult major affective disorder after prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 1997;54(4):322–328. [DOI] [PubMed] [Google Scholar]
- 5.Brown AS, Cohen P, Harkavy-Friedman J, et al. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry 2001;49(6):473–486. doi: 10.1016/S0006-3223(01)01068-X. [DOI] [PubMed] [Google Scholar]
- 6.Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res 2009;204(2):322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, Vinogradov S, Kremen WS, et al. Prenatal Infection and Executive Dysfunction in Adult Schizophrenia. Am J Psychiatry 2009;166(6):683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BK, Magnusson C, Gardner RM, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avramopoulos D, Pearce BD, McGrath J, et al. Infection and inflammation in schizophrenia and bipolar disorder: a genome wide study for interactions with genetic variation. PLoS ONE [Electronic Resour 2015;10(3):e0116696. doi: 10.1371/journal.pone.0116696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmond MM, Montgomery JR, Melnick JL, Cochran GG, Verniaud W. Congenital rubella encephalitis. Effects on growth and early development. Am J Dis Child 1969;118(1):30–31. [PubMed] [Google Scholar]
- 11.Atladottir HO, Thorsen P, Ostergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010;40. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 12.Pratt L, Ni L, Ponzio NM, Jonakait GM. Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6. Pediatr Res 2013;74(4):393–401. doi: 10.1038/pr.2013.126. [DOI] [PubMed] [Google Scholar]
- 13.Theoharides TC, Angelidou A, Alysandratos KD, et al. Mast cell activation and autism. Biochim Biophys Acta 2012;1822(1):34–41. doi: 10.1016/j.bbadis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Angelidou A, Asadi S, Alysandratos KD, Karagkouni A, Kourembanas S, Theoharides TC. Perinatal stress, brain inflammation and risk of autism-review and proposal. BMC Pediatr 2012;12:89. doi: 10.1186/1471-2431-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni L, Acevedo G, Muralidharan B, Padala N, To J, Jonakait GM. Toll-like receptor ligands and CD154 stimulate microglia to produce a factor(s) that promotes excess cholinergic differentiation in the developing rat basal forebrain: implications for neurodevelopmental disorders. Pediatr Res 2007;61(1):15–20. doi: 10.1203/01.pdr.0000249981.70618.18. [DOI] [PubMed] [Google Scholar]
- 16.McAdams RM, Juul SE. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol Res Int 2012:561494. doi: 10.1155/2012/561494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun 2012;26(4):623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, Patterson PH. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain, Behav Immun 2015;46:192–202. doi: 10.1016/j.bbi.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeidán-Chuliá F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JCF. The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev 2014;38:160–172. doi: 10.1016/j.neubiorev.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Meyer U, Nyffeler M, Engler A, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 2006;26(18):4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 2011;71(4):444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 22.Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry 2008;13(2):208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- 23.Dammann O, Leviton A. Intermittent or Sustained Systemic Inflammation (ISSI) and the Preterm Brain. Pediatr Res 2014;75(3):376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik M, Sheikh AM, Wen G, Spivack W, Brown WT, Li X. Expression of inflammatory cytokines, Bcl2 and cathepsin D are altered in lymphoblasts of autistic subjects. Immunobiology 2011;216(1):80–85. doi: 10.1016/j.imbio.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol 2001;120. doi: 10.1016/S0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 26.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2004;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 27.Goeden N, Velasquez J, Arnold KA, et al. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J Neurosci 2016;36(22):6041–6049. doi: 10.1523/JNEUROSCI.2534-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandal MJ, Haney JR, Parikshak NN, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science (80- ) 2018;359(6376):693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res 2011;69(5 Pt 2):26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Textor J, Hardt J, Knuppel S. Dagitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 32.Lash T, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data New York: Springer US; 2009. [Google Scholar]
- 33.Idring S, Rai D, Dal H, et al. Autism Spectrum Disorders in the Stockholm Youth Cohort: Design, Prevalence and Validity. PLoS One 2012;7(7):e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundström S, Reichenberg A, Anckarsäter H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: Prevalence trends over 10 years in general population samples. BMJ 2015;350:1–6. doi: 10.1136/bmj.h1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson R, Carlbring P, Heedman Åsa, Paxling B, Andersson G. Depression, anxiety and their comorbidity in the Swedish general population: point prevalence and the effect on health-related quality of life. Cloninger CR, ed. PeerJ 2013;1:e98. doi: 10.7717/peerj.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med 2013;43(2):239–257. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Os J, Rutten BPF, Poulton R. Gene-Environment Interactions in Schizophrenia: Review of Epidemiological Findings and Future Directions. Schizophr Bull 2008;34(6):1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karl T, Arnold JC. Schizophrenia: a consequence of gene-environment interactions? Front Behav Neurosci 2014;8:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ursini G, Punzi G, Chen Q, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med 2018;(24):792–801. doi: 10.1038/s41591-018-0021-y. [DOI] [PubMed] [Google Scholar]
- 41.Simanek AM, Meier HCS. Association Between Prenatal Exposure to Maternal Infection and Offspring Mood Disorders: A Review of the Literature. Curr Probl Pediatr Adolesc Health Care 2015;45(11):325–364. doi: 10.1016/j.cppeds.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Murphy SK, Fineberg AM, Maxwell SD, et al. Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res 2017;257:102–110. doi: 10.1016/j.psychres.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atladóttir HÓ, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 44.Lee BK, Magnusson C, Gardner RM, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain, Behav Immun 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blomström Å, Karlsson H, Gardner R, Jörgensen L, Magnusson C, Dalman C. Associations Between Maternal Infection During Pregnancy, Childhood Infections, and the Risk of Subsequent Psychotic Disorder—A Swedish Cohort Study of Nearly 2 Million Individuals. Schizophr Bull 2016;42(1):125–133. doi: 10.1093/schbul/sbv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDermott S, Daguise V, Mann H, Szwejbka L, Callaghan W. Perinatal risk for mortality and mental retardation associated with maternal urinary-tract infections. J Fam Pract 2001;50(5):433–437. [PubMed] [Google Scholar]
- 47.Giovanoli S, Engler H, Engler A, et al. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl Psychiatry Psychiatry 2016;6:e772. doi: 10.1038/tp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuillermot S, Luan W, Meyer U, Eyles D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol Autism 2017;8:9. doi: 10.1186/s13229-017-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maršál K, P‐H P, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85(7):843–848. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


