Abstract
PURPOSE:
Increasing use of genomic tumor profiling may blur the line between research and clinical care. We aimed to describe research participants’ perspectives on the purpose of genomic tumor profiling research in pediatric oncology.
METHODS:
We surveyed 45 participants (response rate 85%) in a pilot study of genomic profiling in pediatric solid tumors at four academic cancer centers following return of sequencing results. We defined understanding according to a one-item (“basic”) definition (recognizing that the primary purpose was not to improve the patient’s treatment) and a four-item (“comprehensive”) definition (primary purpose was not to improve patient’s treatment; primary purpose was to improve treatment of future patients; there may not be direct medical benefit; most likely result of participation was not increased likelihood of cure).
RESULTS:
Sixty-eight percent of respondents (30/44) demonstrated basic understanding of the study purpose; 55% (24/44) demonstrated comprehensive understanding. Understanding was more frequently seen in those with higher education and greater genetic knowledge according to basic (81% vs 50%, p=0.05; and 82% vs 46%, p=0.03, respectively) and comprehensive definitions (73% vs 28%, p=0.01; 71% vs 23%, p=0.01). Ninety-three percent of respondents who believed the primary purpose was to improve the patient’s care simultaneously stated that the research also aimed to benefit future patients.
CONCLUSIONS:
Most participants in pediatric tumor profiling research understand that the primary goal of this research is to improve care for future patients, but many express dual goals when participating in sequencing research. Some populations demonstrate increased rates of misunderstanding. Nuanced participant views suggest further work is needed to assess and improve participant understanding, particularly as tumor sequencing moves beyond research into clinical practice.
Keywords: cancer, ethics, genomics, molecular profiling, patient perspectives, pediatric oncology, therapeutic misconception
INTRODUCTION
Parents of children with cancer1,2 and adults with cancer3–5 often fail to understand the purpose of clinical trials in which they participate. Understanding the distinction between the goals of research and clinical care is of particular importance in early-phase oncology trials, in which response rates approximate 10%.6,7 Up to 60% of research participants demonstrate evidence of therapeutic misconception,3,4,8,9 the belief that the primary purpose of research is therapeutic in nature, rather than acquisition of generalizable knowledge.10,11
The precision medicine era invites new exploration of these findings. Paradigm-shifting successes with targeted treatments12–15 highlight the potential of a precision approach to cancer care, as have reports of extraordinary responders among adults16,17 and children.17–20 While advances in targeted therapeutics generate great excitement, they may also blur the line between research and clinical care.21,22 Young adult patients and parents of children with cancer have high hopes/expectations for tumor sequencing,23,24 though only a minority experience clinical benefit.25–29 This mirrors findings among adult cancer patients30–33 and highlights the need for a deeper understanding of the tumor profiling consent process. Though recent work has described genomic knowledge in parents and young adult cancer survivors,23 we know little about how patients and parents undergoing sequencing perceive the role of tumor sequencing research, nor how they conceptualize the balance between research and clinical care in the era of precision cancer medicine.
To better understand patient/parent perceptions of these complex concepts, we queried beliefs of participants in a study involving molecular profiling of pediatric solid tumor samples about the primary purpose of such research.
METHODS
We surveyed consenting participants in the iCat (Individualized Cancer Therapy) pilot study of genomic profiling in children with relapsed, recurrent, and high-risk solid tumors (NCT01853345).25 Participants were approached at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center (Boston, MA), University of California at San Francisco (San Francisco, CA), Columbia University Medical Center (New York, NY), and Children’s National Medical Center (Washington, DC). The study was approved by the Institutional Review Board of all participating institutions.
The Individualized Cancer Therapy (iCat) study
iCat study procedures have been reported previously.25 All patients receiving care at participating institutions were eligible for enrollment if they were ≤30 years at enrollment and had a recurrent, refractory, or high-risk (expected likelihood of cure <25%) extracranial solid tumor with sufficient tumor for submission. The study consent document described the purpose of the study to be “to determine how often the panel of experts can [use tumor sequencing results to] make an individual treatment recommendation,” and to use this information to “help future patients with cancer.” Consent discussions were not standardized, nor was data collected on the content of these discussions.
Enrolled subjects underwent tumor profiling via targeted next generation sequencing and copy number assessment or a Sequenom assay. A multi-disciplinary expert panel reviewed profiling results, utilizing applicable literature to identify results with potential therapeutic implications. A letter was sent to the treating oncologist identifying such results along with variants suggesting a change in diagnosis or possible cancer predisposition syndrome. An “iCat recommendation” was provided for subjects with one or more actionable alterations for which a matched targeted therapy was available via clinical trial or FDA-approved medication; the recommendation described actionable alteration(s) found and strength of evidence for each treatment recommendation.
Study population
iCat participants were offered a self-administered written survey following return of study results to the patient’s oncologist. Surveys were offered in English to the consenting individual: the patient if he/she was ≥18 years at enrollment, or the patient’s parent/guardian, if the patient was <18 at enrollment. Surveys were not offered if: the patient died between the time of enrollment and approach by the study team (N=41); the patient/parent did not understand English sufficiently to complete the survey (N=3); the patient/parent declined further contact from study investigators after enrollment (N=0); and/or the oncologist did not permit approach by the study team (N=4).
Survey methods
Survey procedures have been reported previously.24 Surveys consisted of 103 items and included scales addressing subject understanding of the purpose of clinical research34, genetic knowledge,35 and the SF-36 general health perceptions question. Our primary outcome of interest was participant understanding. Secondary outcomes were participant-level predictors of understanding (demographic characteristics, genetic knowledge, experience with genetics, clinical status, receipt of iCat recommendation/targeted therapy). Eligible subjects were approached at least 4 weeks following return of sequencing results. Participants enrolled between September 2012 and November 2013; surveys were administered between September 2014 and July 2015.
Participant understanding of the purpose of research sequencing
We assessed participant understanding with four independent items (TABLE 1). Three were adapted from the Quality of Informed Consent (QuIC) – a validated measure assessing adult cancer patients’ understanding of the purpose of oncology clinical trials34 and further validated in parents of children with cancer1 – with answer choices of “agree,” “unsure,” and “disagree.”34 The fourth item offered respondents multiple choices regarding their perceived most likely result of study participation.
Table 1.
Survey items for assessment of participant understanding of the purpose of genomic profiling research in pediatric oncology.
| Question stem | Answer choices | |||
|---|---|---|---|---|
| 1) | The main reason this study was done was to improve the treatment of myself/my child | Agree | Unsure* | Disagree |
| 2) | The main reason this study was done was to improve the treatment of future cancer patients | Agree | Unsure* | Disagree |
| 3) | There may not have been direct medical benefit to me/my child from participating | Agree | Unsure* | Disagree |
| 4) | What of the following did you think was most likely to happen because of your participation in this research study? | I/My child would have a better chance of being cured | Doing this testing would give me peace of mind | Doctors would be better able to find cures for future patients |
| Doctors would be able to learn more about my/my child’s cancer | I/my child would have a greater number of treatment options | Nothing was likely to happen as a result of this research | ||
| I would learn about my/my child’s genes | I would learn about my family’s genes | Other | ||
Answer choices coded as indicating participant understanding depicted in boldface (see Figure 1). Answer choices with asterisks excluded from sensitivity analyses (see Appendix Figure A1 and Appendix Table A2)
Participants were asked how well they understood conversation(s) they had with their/their child’s doctor about the iCat study and the testing involved in it, with responses collected on a 5-point Likert scale (extremely well/well/moderately/poorly/extremely poorly). They were also asked to respond to the statement “I feel I have helped myself/my child by participating in this study” (extremely true/very true/somewhat true/a little true/not at all true).
Genetic knowledge/experience
Genetic knowledge was assessed with four items from the Genetic Knowledge Index (GKI) regarding the role of genetics in disease prevention/cure, genetic determinism, heredity, and the role of genetics in health (APPENDIX TABLE A1).35 This validated scale previously has been utilized to measure patient knowledge about genetics/genomics.24,36,37 Respondents were asked if they had regular exposure to genetics and/or genetic information through their job and if they had ever attended any classes/lectures on genes/genetics.
Statistical methods
We defined understanding of the purpose of the study in two ways. “Basic understanding” was defined as accurate recognition that the primary purpose of participation was not to improve their/their child’s treatment (TABLE 1, item 1). “Comprehensive understanding” was defined as understanding all four of the following: 1) the primary purpose was not to improve their/their child’s treatment; 2) the primary purpose was to improve treatment of future patients with cancer; 3) there may not have been direct medical benefit to them/their child; and 4) the most likely result of participation was not an increased likelihood of cure for themselves/their child. Participants who correctly answered all four items were coded as having comprehensive understanding; those who answered zero to three items correctly did not. For example, if a subject identified that the primary purpose of the study was not to improve her child’s treatment, she demonstrated basic understanding of the study’s purpose. If she incorrectly answered any (or all) of the other three understanding items, she did not demonstrate comprehensive understanding. To be as inclusive as possible, and due to the complexity and uncertainty inherent in tumor profiling research, responses of “unsure” to any of the first three items were coded as consistent with understanding. Sensitivity analyses were performed excluding responses of “unsure” from analysis. For the fourth item, only responses that the most likely result of participating in the study was cure were coded as inconsistent with understanding; all other responses were coded as understanding, including answers of “other.” Missing responses to any of the four understanding items were excluded from analysis of comprehensive understanding; only those missing the first item were excluded from analysis of basic understanding.
Self-report of degree of understanding of the consent conversation(s) was dichotomized as “well”/”extremely well” (coded as “good self-reported understanding”) versus all others. Those who answered “extremely true” or “very true” to the item querying how helpful participation was to them/their child were coded as feeling the study to have been helpful, with remaining answer choices coded as feeling it was not.
Experience with genetics was defined as an affirmative response to 1) having regular exposure to genetics or experience with genetics/genetic information, and/or 2) having taken any classes/lectures on genes or genetics. High genetic knowledge was defined as correctly answering all four items from the GKI.35 Those who answered fewer than four GKI items correctly were coded as having low genetic knowledge.
Respondent demographics/characteristics and understanding of results and the purpose of testing were evaluated using descriptive statistics. Bivariable associations between respondent characteristics and understanding of the purpose of tumor profiling were conducted utilizing Fisher’s exact test. Item non-response was <10%, and participants with non-response to an item were excluded from analyses of that item. All analyses were performed using Stata version 13.1 (StataCorp, College Station, TX).
RESULTS
Respondent characteristics
Of 101 subjects who underwent profiling on the iCat study, 53 were eligible for survey administration. Forty-five surveys (85%) were completed. Surveys were completed a median of 13.5 months (interquartile range 11.2–18.8) following return of results to clinicians and 22.6 months (19.1–24.0) following study enrollment. Characteristics of survey respondents are provided in TABLE 2 for the overall cohort and subdivided into patient (24%, N=11) and parent/guardian (76%, N=34) respondents. Characteristics of patients themselves are also provided, subdivided similarly. Sixty-two percent of participants reported having a good understanding of what they were told about the iCat study and its involved testing.
Table 2.
Participant and patient demographics, overall and separately according to whether the survey was completed by the patient’s parent/guardian or by the patient him/herself.
| Overall | Parent/guardian respondent | Patient respondent | |
|---|---|---|---|
| N(%) | N(%) | N(%) | |
| Characteristics of survey respondents | N=45 | N=34 | N=11 |
| Age | |||
| ≥40 | 26 (58) | 26 (76) | 0 (0) |
| <40 | 19 (42) | 8 (24) | 11 (100) |
| Sex | |||
| Male | 18 (40) | 10 (29) | 8 (73) |
| Female | 27 (60) | 24 (71) | 3 (27) |
| Education | |||
| College graduate and higher | 26 (58) | 23 (68) | 3 (27) |
| Less than college graduate | 19 (42) | 11 (32) | 8 (73) |
| Race/ethnicity | |||
| White, non-Hispanic | 25 (56) | 20 (59) | 5 (45) |
| Non-white or Hispanic | 20 (44) | 14 (41) | 6 (55) |
| Experience with genetics and/or genetic testing | |||
| No | 14 (31) | 12 (35) | 2 (18) |
| Yes | 31 (69) | 22 (65) | 9 (82) |
| Genetic knowledge* | |||
| Low genetic knowledge | 13 (32) | 8 (25) | 5 (56) |
| High genetic knowledge | 28 (68) | 24 (75) | 4 (44) |
| Characteristics of patients | |||
| Age | |||
| <2 | 3 (7) | 3 (9) | 0 (0) |
| 2–9 | 15 (33) | 15 (44) | 0 (0) |
| 10–17 | 16 (36) | 16 (47) | 0 (0) |
| ≥18 | 11 (24) | 0 (0) | 11 (100) |
| Sex | |||
| Male | 26 (58) | 18 (53) | 8 (73) |
| Female | 19 (42) | 16 (47) | 3 (27) |
| Diagnosis | |||
| Ewing sarcoma | 5 (11) | 2 (6) | 3 (27) |
| Neuroblastoma | 6 (13) | 5 (15) | 1 (9) |
| Osteosarcoma | 3 (7) | 3 (9) | 0 (0) |
| Renal tumors | 6 (13) | 5 (15) | 1 (9) |
| Rhabdomyosarcoma | 6 (13) | 6 (18) | 0 (0) |
| Other sarcoma | 12 (27) | 7 (21) | 5 (45) |
| Other diagnosis | 7 (16) | 6 (18) | 1 (9) |
| Site | |||
| DFCI | 30 (67) | 25 (74) | 5 (45) |
| Columbia | 4 (9) | 2 (6) | 2 (18) |
| CNMC | 5 (11) | 3 (9) | 2 (18) |
| UCSF | 6 (13) | 4 (12) | 2 (18) |
| Participant-reported health status* | |||
| Excellent/very good | 26 (59) | 22 (67) | 4 (36) |
| Good/fair/poor | 18 (41) | 11 (33) | 7 (64) |
| Participant-reported likelihood of cure | |||
| ≥60% chance | 26 (58) | 21 (62) | 5 (45) |
| <60% chance | 19 (42) | 13 (38) | 6 (55) |
| Receiving treatment at time of survey completion | |||
| No | 24 (53) | 17 (50) | 7 (64) |
| Yes | 21 (47) | 17 (50) | 4 (36) |
| Received iCat treatment recommendation | |||
| No | 33 (73) | 24 (71) | 9 (82) |
| Yes | 12 (27) | 10 (29) | 2 (18) |
| Received targeted treatment | |||
| No | 44 (98) | 33 (97) | 11 (100) |
| Yes | 1 (2) | 1 (3) | 0 (0) |
| Respondent attitudes about iCat study | |||
| Understanding of iCat information | |||
| Poor self-reported understanding | 17 (38) | 12 (35) | 5 (45) |
| Good self-reported understanding | 28 (62) | 22 (65) | 6 (55) |
| Helpfulness of participating in this study | |||
| Not helpful to myself/my child | 25 (56) | 18 (53) | 7 (64) |
| Helpful | 20 (44) | 16 (47) | 4 (36) |
Genetic knowledge was unknown for 4 participants and health status was unknown for 1 participant
Participant understanding
Nearly all survey participants (98%, 44/45) correctly stated that by participating in the study, they were helping doctors and scientists learn information that may benefit future cancer patients, with 89% (39/44) also stating they believed their participation was helping doctors and scientists learn information that may benefit them/their child.
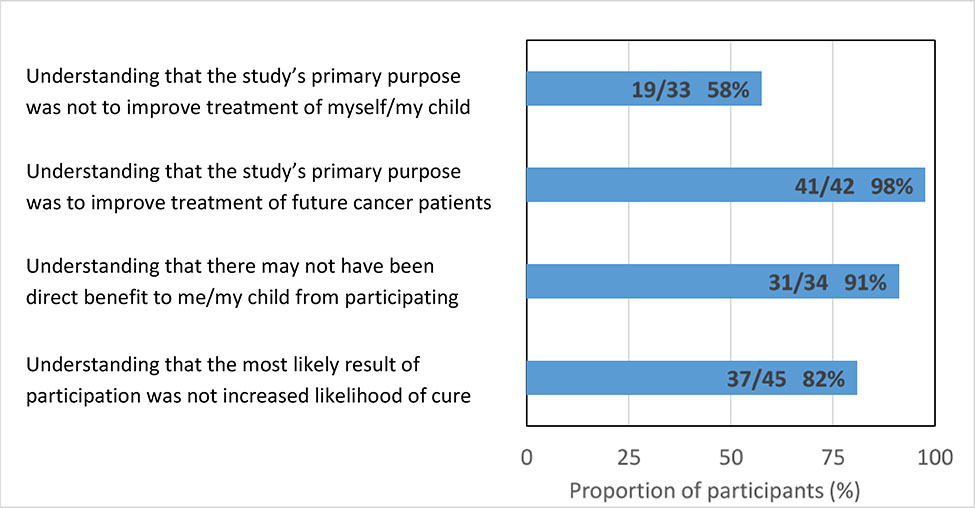
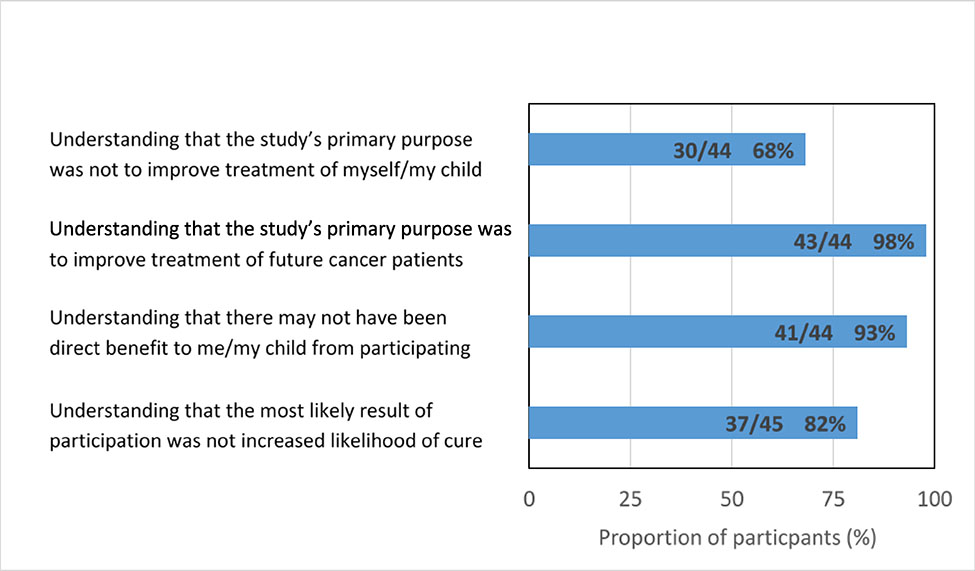
FIGURE 1 depicts participant responses to survey items addressing understanding of the purpose of participating in the iCat research study (data with responses of “unsure” excluded are shown in APPENDIX FIGURE A1). Sixty-eight percent of respondents (30/44) recognized that the primary reason the study was performed was not to improve the treatment of them/their child, which met our definition of basic understanding of the purpose of the study. Fifty-five percent (24/44) demonstrated comprehensive understanding according to the composite four-item definition, including 98% (43/44) who indicated that the primary reason for the study was to improve treatment of future cancer patients, 93% (41/44) who recognized that there may not have been direct benefit to them/their child by participating, and 82% (37/45) who recognized that the most likely result of participation was not a better chance of being cured.
Figure 1.
Participant understanding of the purpose of participation.
Basic understanding was seen more frequently among those with at least a college education (81% vs 50%, p=0.05; TABLE 3), higher genetic knowledge (82% vs 46%, p=0.03), and not receiving cancer-directed therapy at the time of survey completion (83% vs 52%, p=0.05). No significant differences were seen according to respondent age, gender, or race/ethnicity; according to self-reported health status or likelihood of cure; receipt of an iCat treatment recommendation or matched targeted therapy; or to participant-identified understanding of what they were told about the study. Results were similar when responses of “unsure” were excluded from analysis (APPENDIX FIGURE A1).
Table 3.
Relationship between participant demographics and understanding of purpose of research tumor profiling. Values within the table represent frequencies (row percentages).*
| Basic understanding (N=44) | Comprehensive understanding (N=41) | |||
|---|---|---|---|---|
| Characteristics of survey respondents | N (%) | P value | N (%) | P value |
| 30/44 (68%) | 24/44 (55%) | |||
| Age | 0.75 | 0.22 | ||
| ≥40 | 18 (72) | 16 (64) | ||
| <40 | 12 (63) | 8 (42) | ||
| Sex | 0.99 | 0.36 | ||
| Male | 12 (67) | 8 (44) | ||
| Female | 18 (69) | 16 (62) | ||
| Education | 0.05 | 0.01 | ||
| College graduate and higher | 21 (81) | 19 (73) | ||
| Less than college graduate | 9 (50) | 5 (28) | ||
| Race/ethnicity | 0.33 | 0.07 | ||
| White, non-Hispanic | 19 (76) | 17 (68) | ||
| Non-white or Hispanic | 11 (58) | 7 (37) | ||
| Experience with genetics and/or genetic testing | 0.32 | 0.34 | ||
| No | 8 (57) | 6 (43) | ||
| Yes | 22 (73) | 18 (60) | ||
| Genetic knowledge* | 0.03 | 0.01 | ||
| Low genetic knowledge | 6 (46) | 3 (23) | ||
| High genetic knowledge | 23 (82) | 20 (71) | ||
| Survey completed by | 0.29 | 0.08 | ||
| Parent/guardian | 24 (73) | 21 (64) | ||
| Patient | 6 (55) | 3 (27) | ||
| Characteristics of patients | ||||
| Participant-reported health status* | 0.99 | 0.99 | ||
| Excellent/very good | 17 (65) | 14 (54) | ||
| Good/fair/poor | 12 (71) | 9 (53) | ||
| Participant-reported likelihood of cure | 0.21 | 0.37 | ||
| ≥60% chance | 15 (60) | 12 (48) | ||
| <60% chance | 15 (79) | 12 (63) | ||
| Receiving treatment at time of survey completion | 0.05 | 0.23 | ||
| No | 19 (83) | 15 (65) | ||
| Yes | 11 (52) | 9 (43) | ||
| Received iCat treatment recommendation | 0.72 | 0.33 | ||
| No | 21 (66) | 19 (59) | ||
| Yes | 9 (75) | 5 (42) | ||
| Received targeted treatment | 0.99 | 0.99 | ||
| No | 29 (67) | 23 (53) | ||
| Yes | 1 (100) | 1 (100) | ||
| Respondent attitudes about iCat study | ||||
| Understanding of iCat information | 0.18 | 0.12 | ||
| Poor self-reported understanding | 14 (82) | 12 (71) | ||
| Good self-reported understanding | 16 (59) | 12 (44) | ||
| Helpfulness of participating in the study | 0.11 | 0.03 | ||
| Not helpful to myself/my child | 19 (79) | 17 (71) | ||
| Helpful | 11 (55) | 7 (35) | ||
Genetic knowledge was unknown for 3 participants and health status was unknown for 1 participant
Similar results were seen with understanding defined by the composite four-item scale. Comprehensive understanding of the purpose of genomic profiling research was seen with statistically greater frequency among those with at least a college education (73% vs 28%, p=0.01) and higher genetic knowledge (71% vs 23%, p=0.01), and among white/non-Hispanic respondents (68% vs 37%, p=0.07), though the latter was not statistically significant. Statistically significant differences in respondent understanding were not seen according to respondent age or gender, or self-reported health status or likelihood of cure. Similarly, no statistical difference in understanding was seen according to receipt of an iCat treatment recommendation or matched targeted therapy, or according to whether the respondent reported a good understanding of what they were told about the study/testing. Decreased understanding was seen in those who stated participating in the study had been helpful to them/their child (35% vs 71%, p=0.03). Sensitivity analyses excluding responses of “unsure” provided similar findings (APPENDIX TABLE A2). Time between return of results and survey completion did not differ statistically between participants with and without basic understanding (median 13.3 vs 16.0 months, p=0.31) or comprehensive understanding (median 13.2 vs 15.0 months, p=0.34).
Many participants recognized dual roles for this study. Among those who mistakenly identified the primary purpose as improving their/their child’s treatment, 93% (13/14) simultaneously recognized that it aimed to benefit future patients. 93% (13/14)_of this subgroup also correctly reported that they/their child might not have directly benefited from participating. All respondents who stated that the most likely result of participation was increased chance of cure also identified benefiting future patients as the study’s primary purpose. 28% (12/43) of those who identified that the primary purpose was to benefit future patients also reported that the primary purpose was to improve their/their child’s treatment.
DISCUSSION
In this multi-institutional study examining the role of molecular profiling of pediatric solid tumors, nearly all participants recognized that the primary purpose was to benefit future patients. However, approximately one-third of respondents believed that the primary purpose of the trial was to improve their/their child’s treatment, and nearly one-fifth expected participation to impart a greater chance of cure.
Although these responses raise concerns about the quality of informed consent for tumor sequencing, they must be considered in context of a complex technology with an evolving role in clinical care. Importantly, participants often felt that sequencing had dual roles, with potential benefits to future patients but also to themselves/their children. This duality is echoed by the American Society of Clinical Oncology, which states that early phase clinical trials in oncology simultaneously generate new knowledge and provide participants the opportunity for psychological and clinical benefit.22,38 Oncologists often balance dual goals for patients: recommending enrollment in a phase I trial while hoping for patient benefit, or simultaneously providing palliative and cancer-directed (“blended”) care.39 In the era of precision cancer medicine, it is reasonable that patients/families might perceive such dualities as well.
This duality has important clinical implications. If patients/parents frequently identify dual goals when participating in sequencing research, consenting clinicians should query and explore these goals during pre-sequencing counseling. Further work is needed to better understand how participants conceptualize and balance dual goals in genomics research. However, an initial approach could be to discuss the primary goal of the study as gaining new knowledge to help future patients, followed by acknowledging that many patients/parents—and many clinicians—hold hope that the child will also benefit from participation, while tempering this statement with realistic expectations. In the case of next-generation sequencing, for example, it is important to note that the number of patients who experience direct benefit via receipt of a targeted therapy is quite low, likely in the range of 3–19%.25–29
Our results also underscore the significance of hope among patients and parents of children with cancer in clinical and research settings.40–42 Hopeful thinking may partially explain why participants who felt the study had helped them/their child and those who were receiving cancer-directed therapy at the time of survey completion less frequently demonstrated understanding of the purpose of research tumor sequencing.
In this cohort, understanding was observed more frequently in those with at least a college education and those with good genetic knowledge. This finding, also reported elsewhere,23 is not surprising given the complexity of these concepts and the expected link between understanding and health literacy/numeracy.43 Understanding also varied according to race/ethnicity, consistent with similar work in the pediatric oncology phase I literature,2 although not reaching statistical significance in this pilot study. These disparities underscore the importance of attention to the needs of vulnerable populations when counseling about genomic results, though the optimal mechanism for such counseling remains unclear.44
Prior work in pediatric oncology has identified that refinement of the consent process may improve understanding,45 but optimal strategies to adequately convey the complexities of tumor sequencing and support fully informed consent for participation in sequencing research are not yet known. A follow-up study is in development to examine the benefit of such an intervention for those who demonstrate less than comprehensive understanding as defined in this cohort. Tools such as these will become only more important as genomic sequencing becomes more frequently used in the clinical setting and research explores the role of RNA sequencing, methylation profiling, or the next promising precision modality.
Data collected in this study are limited primarily by the cross-sectional nature and timing of survey administration. Patients/parents may have better understood the purpose of profiling closer to the time of consent, though understanding did not vary statistically with time to survey completion in this cohort. Some may also disagree with how we have defined “understanding” in this work. Individual respondents may have felt the primary purpose of the study for them was different than it was for the researchers, for example. We consider our definitions to be a starting point for clarifying the complex issues inherent in studies of pediatric tumor profiling. Our use of validated items to define understanding1,34 and our similar results for both basic and comprehensive understanding support these definitions. Further, variability in consent discussions could have impacted participant understanding of the study’s purpose. Additional work is necessary to isolate the role of these important considerations.
Respondents were queried after return of sequencing results, which could have affected their responses. Many study participants died before surveys could be administered; however, demographic and clinical characteristics of respondents mirror those of the overall cohort.25 Finally, subjects were enrolled at four large academic centers, so results may not be generalizable to those from smaller and/or community centers. This could, for example, explain the unexpectedly high genetic knowledge and experience seen in this cohort.
Although some participants misidentify the primary goal of tumor profiling research as therapeutic in nature, participants’ views are nuanced. Nonetheless, some populations demonstrate decreased understanding of the purpose of tumor profiling research and warrant special attention to ensure equitably informed consent for all research subjects. Interventional work aimed at improving participant understanding of these complexities and nuances is necessary as sequencing moves from the laboratory to the clinic. Such work can guide pediatric oncologists how to manage expectations and best counsel patients and families about the meaning and significance of clinical profiling results.
KEY POINTS.
Key objective:
How well do participants in a pediatric genomic tumor profiling research study understand the purpose of participating in such research?
Knowledge generated:
Most participants recognize that the purpose of such research is to benefit future patients, but many participants demonstrate some degree of misunderstanding about the purpose of this research and some subgroups demonstrate increased rates of misunderstanding. Further, many participants simultaneously identify dual purposes for genomic tumor profiling research in pediatric oncology.
Relevance:
Consenting clinicians should query and explore participant goals during pre-sequencing counseling, identifying both 1) those who do not recognize that the primary purpose of research sequencing is to generate knowledge to help future patients, and 2) those who report dual purposes for this research. Further work is necessary to better understand the perspectives and motivations of those expressing this duality and to develop and test interventions aimed at improving equitable understanding of the purpose of genomic tumor profiling research in pediatric oncology.
ACKNOWLEDGEMENTS
We are indebted to the patients and parents who participated in this study, to Shelly Allen, Amanda Mahoney, and Danielle Pendrick for their assistance with enrollment of participants and survey administration, and to Erin Parker for administrative and data management support.
This work was supported by Hyundai Hope on Wheels (KAJ, JGB), the Friends for Life Foundation (KAJ), the Gillmore Fund (KAJ), and National Institutes of Health Training Grant 5T32 CA136432 (JMM), and support from Pedals for Pediatrics (JMM), the Harvard Medical School Center for Bioethics (JMM), and the Dana-Farber Cancer Institute Division of Population Sciences (JMM).
ABBREVIATIONS:
- iCat
Individualized Cancer Therapy
- QuIC
Quality of Informed Consent
- GKI
Genetic Knowledge Index
Appendix
Figure A1.
Participant understanding of the purpose of participation (“unsure” responses excluded).
Table A1.
Survey items for assessment of participant genetic knowledge.
| Question stem | Answer choices | |
|---|---|---|
| Once a genetic marker for a disorder is identified in a person, the disorder can be prevented or cured. | True | False |
| If a person has a genetic marker for a disorder, the person will always get the disorder. | True | False |
| Only mothers can pass on genetic disorders. | True | False |
| People who have a genetic marker for a disease are unhealthy. | True | False |
Correct answer choices depicted in boldface. All items adapted from the Genetic Knowledge Index (GKI).35
Table A2.
Sensitivity analyses for the relationship between participant demographics and understanding of the purpose of research tumor profiling (“unsure” responses excluded). Values within the table represent frequencies (row percentages).*
| Basic understanding (N=33) | Comprehensive understanding (N=26) | |||
|---|---|---|---|---|
| Characteristics of survey respondents | N (%) | P value | N (%) | P value |
| 19/33 (58%) | 16/26 (62%) | |||
| Age | 0.27 | 0.99 | ||
| ≥40 | 14 (67) | 12 (63) | ||
| <40 | 5 (42) | 4 (57) | ||
| Sex | 0.72 | 0.66 | ||
| Male | 6 (50) | 4 (50) | ||
| Female | 13 (62) | 12 (67) | ||
| Education | 0.03 | 0.19 | ||
| College graduate and higher | 15 (75) | 13 (72) | ||
| Less than college graduate | 4 (31) | 3 (38) | ||
| Race/ethnicity | 0.30 | 0.42 | ||
| White, non-Hispanic | 12 (67) | 11 (69) | ||
| Non-white or Hispanic | 7 (47) | 5 (50) | ||
| Experience with genetics and/or genetic testing | 0.46 | 0.37 | ||
| No | 5 (45) | 3 (43) | ||
| Yes | 14 (64) | 13 (68) | ||
| Genetic knowledge* | 0.02 | 0.16 | ||
| Low genetic knowledge | 3 (30) | 2 (33) | ||
| High genetic knowledge | 16 (76) | 14 (70) | ||
| Survey completed by | 0.11 | 0.99 | ||
| Parent/guardian | 17 (65) | 14 (61) | ||
| Patient | 2 (29) | 2 (67) | ||
| Characteristics of patients | ||||
| Participant-reported health status | 1.00 | 0.42 | ||
| Excellent/very good | 11 (55) | 10 (56) | ||
| Good/fair/poor | 8 (62) | 6 (75) | ||
| Participant-reported likelihood of cure | 0.16 | 0.25 | ||
| ≥60% chance | 8 (44) | 7 (50) | ||
| <60% chance | 11 (73) | 9 (75) | ||
| Receiving treatment at time of survey completion | 0.29 | 0.23 | ||
| No | 10 (71) | 10 (77) | ||
| Yes | 9 (47) | 6 (46) | ||
| Received iCat treatment recommendation | 0.99 | 0.64 | ||
| No | 15 (58) | 13 (65) | ||
| Yes | 4 (57) | 3 (50) | ||
| Received targeted treatment | 0.99 | 0.99 | ||
| No | 18 (56) | 15 (60) | ||
| Yes | 1 (100) | 1 (100) | ||
| Respondent attitudes about iCat study | ||||
| Understanding of iCat information | 0.09 | 0.22 | ||
| Poor self-reported understanding | 10 (77) | 8 (80) | ||
| Good self-reported understanding | 9 (45) | 8 (50) | ||
| Helpfulness of participating in iCat study | 0.30 | 0.23 | ||
| Not helpful to myself/my child | 11 (69) | 10 (77) | ||
| Helpful | 8 (47) | 6 (46) | ||
For the analysis of “basic understanding,” genetic knowledge was unknown for 2 participants
References
- 1.Truong TH, Weeks JC, Cook EF, et al. : Outcomes of informed consent among parents of children in cancer clinical trials. Pediatr Blood Cancer 57:998–1004, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Cousino MK, Zyzanski SJ, Yamokoski AD, et al. : Communicating and understanding the purpose of pediatric phase I cancer trials. J Clin Oncol 30:4367–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joffe S, Cook EF, Cleary PD, et al. : Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet 358:1772–7, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Pentz RD, White M, Harvey RD, et al. : Therapeutic misconception, misestimation, and optimism in participants enrolled in phase 1 trials. Cancer 118:4571–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinfurt KP, Seils DM, Lin L, et al. : Research participants’ high expectations of benefit in early-phase oncology trials: are we asking the right question? J Clin Oncol 30:4396–400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horstmann E, McCabe MS, Grochow L, et al. : Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med 352:895–904, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Italiano A, Massard C, Bahleda R, et al. : Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Ann Oncol 19:787–92, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Appelbaum PS, Lidz CW, Grisso T: Therapeutic misconception in clinical research: frequency and risk factors. IRB 26:1–8, 2004 [PubMed] [Google Scholar]
- 9.Appelbaum PS, Anatchkova M, Albert K, et al. : Therapeutic misconception in research subjects: development and validation of a measure. Clin Trials 9:748–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lidz CW, Appelbaum PS: The therapeutic misconception: problems and solutions. Med Care 40:V55–63, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Henderson GE, Churchill LR, Davis AM, et al. : Clinical trials and medical care: defining the therapeutic misconception. PLoS Med 4:e324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Druker BJ, Sawyers CL, Kantarjian H, et al. : Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 344:1038–42, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian HM, Shah NP, Cortes JE, et al. : Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 119:1123–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwak EL, Bang YJ, Camidge DR, et al. : Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363:1693–703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon BJ, Mok T, Kim DW, et al. : First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–77, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Wagle N, Grabiner BC, Van Allen EM, et al. : Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov 4:546–53, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drilon A, Laetsch TW, Kummar S, et al. : Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 378:731–739, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushner BH, Cheung NV, Modak S, et al. : A phase I/Ib trial targeting the Pi3k/Akt pathway using perifosine: Long-term progression-free survival of patients with resistant neuroblastoma. Int J Cancer 140:480–484, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heritier S, Jehanne M, Leverger G, et al. : Vemurafenib Use in an Infant for High-Risk Langerhans Cell Histiocytosis. JAMA Oncol 1:836–8, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Laetsch TW, DuBois SG, Mascarenhas L, et al. : Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 19:705–714, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimmelman J: Is Participation in Cancer Phase I Trials Really Therapeutic? J Clin Oncol. 35:135–138, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber JS, Levit LA, Adamson PC, et al. : Reaffirming and Clarifying the American Society of Clinical Oncology’s Policy Statement on the Critical Role of Phase I Trials in Cancer Research and Treatment. J Clin Oncol 35:139–140, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberg JA, Ruiz J, Ali-Shaw T, et al. : Whole-Genome and Whole-Exome Sequencing in Pediatric Oncology: An Assessment of Parent and Young Adult Patient Knowledge, Attitudes, and Expectations. JCO Precision Oncology 2:1–11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marron JM, DuBois SG, Glade Bender J, et al. : Patient/parent perspectives on genomic tumor profiling of pediatric solid tumors: The Individualized Cancer Therapy (iCat) experience. Pediatr Blood Cancer 63:1974–82, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris MH, DuBois SG, Glade Bender JL, et al. : Multicenter Feasibility Study of Tumor Molecular Profiling to Inform Therapeutic Decisions in Advanced Pediatric Solid Tumors: The Individualized Cancer Therapy (iCat) Study. JAMA Oncol 2:608–615, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Mody RJ, Wu YM, Lonigro RJ, et al. : Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 314:913–25, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons DW, Roy A, Yang Y, et al. : Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol 2:616–624, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang W, Brohl AS, Patidar R, et al. : MultiDimensional ClinOmics for Precision Therapy of Children and Adolescent Young Adults with Relapsed and Refractory Cancer: A Report from the Center for Cancer Research. Clinical Cancer Research 22:3810–3820, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harttrampf AC, Lacroix L, Deloger M, et al. : Molecular Screening for Cancer Treatment Optimization (MOSCATO-01) in Pediatric Patients: A Single-Institutional Prospective Molecular Stratification Trial. Clinical Cancer Research 23:6101–6112, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Gollust SE, Gordon ES, Zayac C, et al. : Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics 15:22–30, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller FA, Hayeems RZ, Bytautas JP, et al. : Testing personalized medicine: patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. Eur J Hum Genet 22:391–5, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchette PS, Spreafico A, Miller FA, et al. : Genomic testing in cancer: patient knowledge, attitudes, and expectations. Cancer 120:3066–73, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703–713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joffe S, Cook EF, Cleary PD, et al. : Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst 93:139–47, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Furr LA, Kelly SE: The Genetic Knowledge Index: developing a standard measure of genetic knowledge. Genet Test 3:193–9, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Gray SW, Park ER, Najita J, et al. : Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: results from the CanSeq study. Genet Med 18:1011–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carere DA, Couper MP, Crawford SD, et al. : Design, methods, and participant characteristics of the Impact of Personal Genomics (PGen) Study, a prospective cohort study of direct-to-consumer personal genomic testing customers. Genome Med 6:96, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber JS, Levit LA, Adamson PC, et al. : American Society of Clinical Oncology policy statement update: the critical role of phase I trials in cancer research and treatment. J Clin Oncol 33:278–84, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrell BR, Temel JS, Temin S, et al. : Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 35:96–112, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Kamihara J, Nyborn JA, Olcese ME, et al. : Parental hope for children with advanced cancer. Pediatrics 135:868–74, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Miller VA, Cousino M, Leek AC, et al. : Hope and persuasion by physicians during informed consent. J Clin Oncol 32:3229–35, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marron JM, Cronin AM, Kang TI, et al. : Intended and unintended consequences: Ethics, communication, and prognostic disclosure in pediatric oncology. Cancer 124:1232–1241, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.French M, Institute of Medicine (U.S.). Roundtable on Health Literacy: Health literacy and numeracy : workshop summary. Washington, D.C., National Academies Press, 2014 [PubMed] [Google Scholar]
- 44.Genetic counseling: an indispensable step in the genetic testing process. J Oncol Pract 4:96–8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberg JA, Glade Bender JL, Cohn EG, et al. : Overcoming challenges to meaningful informed consent for whole genome sequencing in pediatric cancer research. Pediatr Blood Cancer 62:1374–80, 2015 [DOI] [PubMed] [Google Scholar]