Abstract
Objective:
A previous genome-wide association study linked overexpression of an ATP-binding cassette transporter, ABCC5, in humans with a susceptibility to developing type 2 diabetes (T2D) with age (1). Specifically, ABCC5 gene overexpression was shown to be most strongly associated with increased visceral fat mass and reduced peripheral insulin sensitivity. Currently, the role of ABCC5 in diabetes and obesity is unknown. This study reports the metabolic phenotyping of a global Abcc5 knock-out mouse.
Methods:
A global Abcc5−/− mouse was generated by CRISPR/Cas9. Fat mass were determined by weekly EchoMRI and fat pads dissected and weighed at week 18. Glucose homeostasis was ascertained by an oral glucose tolerance test (OGTT), intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test (ITT). Energy expenditure and locomotor activity were measured using PhenoMaster cages. GLP-1 levels in plasma, primary gut cell cultures and GLUTag cells were determined by ELISA.
Results:
Abcc5−/− mice had decreased fat mass, increased plasma levels of GLP-1, were more insulin sensitive and more active. Recombinant overexpression of ABCC5 protein in GLUTag cells decreased GLP-1 release.
Conclusions:
ABCC5 protein expression levels are inversely related to fat mass and appears to play a role in the regulation of GLP-1 secretion from enteroendocrine cells.
Keywords: ABCC5, MRP5, GLP-1, L-cell, insulin sensitivity
INTRODUCTION
Hormones secreted by enteroendocrine cells, the endocrine cells of the gut, are central to the regulation of gastrointestinal physiology, energy metabolism and appetite (2). In particular, the central role of gut hormone action on energy metabolism has been demonstrated by bariatric surgery outcomes, which show that increased gut hormone release contributes to the reversal of pathological insulin resistance in type 2 diabetics (T2D) within days following Roux-en-Y bypass surgery (3–5). The incretin hormone glucagon-like peptide 1 (GLP-1), which potentiates insulin release from pancreatic β-cells, plays a pivotal role in the changes observed in glucose handling post surgery (6, 7). GLP-1 has multiple actions on peripheral tissues, for example, driving cell proliferation in the pancreas and being both anti-apoptotic and neuroprotective (8). GLP-1 receptor (GLP-1R) activation leads to enhanced satiety, weight loss, decreased glucose production in the liver and enhanced insulin sensitivity in skeletal muscle; with a GLP-1R agonists currently in clinical use for the treatment of T2D (8).
A recent genome-wide association study (GWAS) linked elevated expression of an ATP-binding cassette transporter, ABCC5, in subcutaneous adipose tissue to reduced peripheral insulin sensitivity in nondiabetic individuals with associated increased visceral fat accumulation and a 3-fold increased risk of developing T2D with age (1). This trend was observed in populations of disparate ancestry. The role of ABCC5 in diabetes and obesity remains unexplored and any link between ABCC5 overexpression and increased fat stores is unknown.
The ABC transporters are a large family of membrane ATPases best known for their roles in multi-drug resistance observed in chemotherapy-resistant tumours (9). However, these transporters fulfil many other essential functions such as antigen presentation to the immune system (TAP1/ABCB2) (10, 11), Cl- ion permeability of the cell membrane (CFTR/ABCC7) (12), and the regulation of insulin release by adenosine nucleotides and sulphonyl urea drugs from pancreatic β-cells by the sulphonyl urea receptor (SUR1/ABCC8) which forms the KATP channel complex along with Kir6.2 (13–15). The role of ABCC5 transporter activity in mammals is currently unknown, but knocking Abcc5 gene expression out in animal models indicated a role for this protein in heme transport in C.elegans and hind gut formation in sea urchins (16, 17). Jansen et al. demonstrated that ABCC5 is a glutamate conjugate transporter and Abcc5−/− mouse tissues were shown to accumulate up to 8 different glutamate metabolites, including the inhibitory neuropeptides N-acetylaspartylglutamate (NAAG) and N-acetylaspartyldiglutamate (NAAG2) (18).
This study reports the metabolic phenotyping of Abcc5 knock out mice, Abcc5-/−. Our work demonstrated that ABCC5 protein expression plays a central role in energy metabolism in mammals, with Abcc5−/− mice showing lower white and brown adipose tissue and increased GLP-1 release from enteroendocrine cells of the small intestine.
METHODS
CRISPR/Cas9 ABCC5 knockout mice
The Abcc5−/− (knock out) CRISPR/Cas9 mice were generated on a B6N background and were obtained from the MRC Harwell Institute, which distributes these mice on behalf of the European Mouse Mutant Archive (www.infrafrontier.eu). The protospacer sequences used to knock out the Abcc5 gene through direct injection into blastocysts were: Abcc5_5’: 5’-GCTGTGGGTTGCTGATTGCA GGG-3’ and Abcc5_3’: 5’-CTTCTCTCACACATAGCCAAAGG-3’.
Metabolic phenotyping
All animal studies were approved by the Medical Research Council Harwell Institute Ethical Review Committee, and all procedures were carried out within license restrictions (PPL 30/3146) under the Animal (Scientific Procedures) Act 1986, issued by the UK Government Home Office Department. Abcc5−/− mice and wild-type (wt) littermate controls were kept in accordance with Home Office welfare guidance (12 hours light- and dark cycle; temperature 21°C±2°C, and humidity 55%±10% at the Mary Lyon Centre animal facility, MRC Harwell, UK. Mice had free access to water (10ppm chlorine) and were fed ad libitum on standard chow (RM3; Special Diet Services, Essex, United Kingdom). All in vivo studies were performed on mice aged 10–18 weeks.
Body mass and composition
Body mass was measured for two independent cohorts of mice at baseline (week 4) and weekly thereafter on scales calibrated to 0.01g. Whole body composition (fat and lean mass) was determined using an EchoMRI™−136 Body Composition Analyser for Live Small Animals (Echo Medical Systems, Houston, TX) at baseline (week 4) and weekly thereafter for Abcc5−/− mice and wild-type (wt) littermate controls.
Glucose Tolerance Tests
Oral glucose tolerance test (OGTT):
12 week old Abcc5−/− mice and wild-type (wt) littermate controls were fasted overnight (16hrs). The mice were weighed and fasting glucose levels measured from whole blood via tail bleed under local anaesthesia (5% EMLA cream, Eutectic Mixture of Local Anesthetics, Lidocaine/Prilocaine, AstraZeneca, UK). An oral gavage of 20% glucose solution in 0.9% NaCl at 2g/kg of body mass was administered and whole blood glucose measurement taken at 15min, 30min, 60min and 120min after the gavage. The glucose measurements were performed by using a hand-held AlphaTRAK glucometer for pets (Abbott Laboratories).
Intraperitoneal glucose tolerance test (IPGTT):
13 week old Abcc5−/− mice and wild-type (wt) littermates were processed as for OGTT. Intraperitoneal injections of 20% glucose solution in 0.9% NaCl at 2g/kg of body mass were administered and whole blood glucose samples taken at 15min, 30min, 60min and 120min via tail bleed.
Insulin Tolerance Test (ITT)
At week 14, Abcc5−/− mice and wild-type littermate controls of were fasted for 4 hours during the light phase. The mice were weighed, and whole blood samples collected at time 0 (t0) via tail vein for baseline glucose measurements. Intraperitoneal (IP) injections of 0.5iU/kg mouse (females) or 1.0iU/kg mouse (males) of insulin diluted in 0.9% NaCl in sterile water were made, and subsequent blood samples were taken at 15min, 30min, 45min, 60min and 90min. Blood glucose uptake measurements were taken using AlphaTrak glucometer (Abbott Laboratories).
Indirect calorimetry (energy expenditure, locomotor activity)
At week 12, the Abcc5−/− mice and wild-type littermate controls were individually housed in PhenoMaster cages (TSE Systems, Bad Homburg, Germany) for collection of energy intake/expenditure related data over 24 hours. The cage system included photo beam-based activity monitoring that records ambulatory movements in the horizontal and vertical planes. An indirect gas calorimetry system simultaneously measured oxygen consumption (VO2), carbon dioxide production (VCO2) and respiratory exchange ratio (RER).
Adipose tissue harvest and Western Blots.
Epididymal white adipose tissue (epiWAT), periovarian white adipose tissue (periWAT) and interscapular brown adipose tissue (iBAT) were collected as described before (19) and immediately weighed. All tissues were snap-frozen in liquid nitrogen and stored at −80°C. Tissue processing is detailed in Supplementary Information.
siRNA Abcc5 gene knock-down and recombinant ABCC5 protein overexpression in GLUTag cells
GLUTag cells were used with permission of Prof Daniel Drucker (Toronto). Cells were maintained in low glucose DMEM medium (11885084, Gibco) supplemented with 2mM glutamine (G7513–100ML Sigma), 100U/mL Penicillin and 100ug/mL Streptomycin (15140122, Gibco) and 10% Foetal Bovine Serum (F7524–500ML, Sigma) in tissue culture flasks coated with 0.4% Matrigel® (354234, Corning). For knock-down and overexpression experiments cells were seeded onto 24-well plates coated with 0.4% Matrigel® at the density of 0.05 × 106 cells per well and incubated until 80% confluent. Abcc5 siRNA knock-down were performed with pre-designed siRNA (AM16708, Ambion) targeting reference Abcc5 sequence (probe ID: 188572). Abcc5 knock-down was achieved by Lipofectamine RNAiMAX Transfection Reagent (13778075, Life Technologies) according to the manufacturer’s protocol. Non-targeting siRNA (AM4611, Ambion) was used as a negative control. ABCC5 protein overexpression was achieved by the bPEI (408727–100ML, Sigma) mediated transfection of the mammalian expression vector (pSF-CMV-Amp, Oxford Genetics) carrying a codon-optimised human ABCC5 gene sequence (custom synthesis, GenScript). Empty vector pSF-Amp-CMV was used as a negative control and no effect on the expression of ABCC5 was observed. ABCC5 gene expression was quantified by RT-qPCR (Primers were: Abcc5, fwd CCTGCTGCGTCACTGTAAGA, rev TCAAACTCCACCACCTGTCC; pSF-CMV-Amp-ABCC5, fwd CAGCGTATCTCTCTGGCTCG, rev AGCACGGTCTTGGACTTCAG) followed by the protein analysis on Western blot (antibodies were goat polyclonal anti-ABCC5, sc-5781, Santa Cruz Biotechnology, 1:200; secondary goat anti-Rabbit IgG (H/L):HRP, STAR124P, AbD Serotec, 1:10,000).
Glucagon-like peptide-1 (GLP-1) secretion assay
GLP-1 secretion assay was similar for GLUTag cells and primary small intestine cultures. Cells were grown on Matrigel-coated 24-well plates until 80% confluent (GLUTag cells) or overnight (gut primary cultures). Cells were washed twice with warm PBS and then treated with different glucose concentrations (0mM, 1mM, 6mM) prepared in Krebs buffer (138mM NaCl, 4.5mM KCl, 2.5mM CaCl2, 1.2mM MgCl2, 4.2mM NAHCO3, 1.2mM Na2HPO4/NaH2PO4, 10mM HEPES), supplemented with 0.1% fatty-acid free BSA (A6003–10G, Sigma), and incubated for 2hrs at 37°C, 5% CO2. Supernatants were harvested into the Triton-X100/Tween-20 mixture (0.05% and 0.04% final concentrations, respectively) and spun down at 300xg for 5mins to remove floating cells. The remaining cell monolayer was lysed in RIPA buffer. Supernatants were analysed for active GLP-1 by ELISA. GLP-1 secretion from GLUTag cells was measured by two independent ELISA kits (EZGLPHS-35K, Millipore and 62GLPPEG, Cisbio). Active GLP-1 secretion from primary gut cultures was measured by FRET-based ELISA (62GLPPEG, Cisbio). All buffer reagents were from Sigma, and the glucose solution (A2494001) and HEPES buffer (15630056) were from Life Technologies.
Isolation and culture of gut primary cells from Abcc5−/− mice
Mixed primary cultures of murine intestine isolated from Abcc5−/− mice and their littermate wild-type controls were prepared as previously described and is detailed in Supplementary Information (20).
Blood chemistry analysis
Total cholesterol, HDL, LDL, glycerol and triglyceride levels were determined in terminal lithium-heparin plasma samples collected at 5min following an oral glucose gavage of overnight-fasted Abcc5−/− mice and their littermate wild-type controls, using a Beckman Coulter AU680 clinical chemistry analyser with reagents and settings recommended by the manufacturer.
Abcc5 gene expression analysis
ABC transporter expression profiles were determined by RNA-seq as previously described (21). Briefly, fluorescent and non-fluorescent cells were isolated in triplicate by FACS from the duodenum/jejunum (top 10cm of the small intestine (SI), ileum (bottom 10cm SI) and colon of NeuroD1-CrexRosa26EYFP or GLU-Venus mice, labelling all EECs or only proglucagon-expressing cells, respectively. Total RNA (isolated with RNeasy Micro Plus kit (QIAGEN) and amplified using Ovation RNA-seq System V2 (NuGEN)) was used to create barcoded libraries, which were sequenced using an Illumina HiSeq 2500 system at the Genomics Core Facility, Cancer Research UK Cambridge Institute. Sequence reads were demultiplexed using the Casava pipeline (Illumina) and then aligned to the mouse genome (GRCm38) using Tophat version 2.1.0 (http://ccb.jhu.edu/software/tophat/index.shtml ). Gene expression (FPKM) was determined using Cufflinks version 2.2.1 (http://cole-trapnell-lab.github.io/cufflinks/) and differential gene expression was assessed by DESeq2 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html), excluding one GLU-Venus duodenal fluorescently labelled data-set due to apparent contamination.
Metabolomics
Metabolite extraction from cells and Mass spectrometry.
Metabolites were extracted from approximately 5×106 cells (grown in cell culture dishes) by addition of 500 μL of ice cold 80% aqueous methanol. Supernatants were combined and filtered using a 3 kDa ultrafilter (Millipore), dried in a SpeedVac and subsequently stored at −80°C. On the day of analysis, the dried extracts were re-constituted in 60 μL of ice cold 80% aqueous methanol. A quality control (QC) sample was made by combing 5 μL of each sample. Sample analysis was performed using anion-exchange chromatography coupled directly to a high resolution orbitrap mass spectrometer as previously described and is detailed in Supplementary Information (22).
Statistics
Data are presented as mean +/− SEM. Simple pairwise comparisons were made using unpaired 2-tailed t-tests. For sample numbers ≥10–15 a Student’s t-test was used. For sample numbers ≤10 or where unequal numbers of two groups were compared, the more stringent Welch’s unequal variances t-test was used. Multiple comparisons were made using one- or two-way ANOVA with a Bonferroni post-hoc test. A P value <0.05 for a 95% confidence interval was regarded as statistically significant. Statistics were performed using GraphPad Prism6 (Graphpad Software, CA, USA).
RESULTS
Abcc5−/− mice have lower body mass, due to decreased adiposity.
At 16 weeks, both female and male Abcc5−/− mice weighed ∼10% less than wild-type (wt) littermates with a difference in body mass at 16 weeks of 2.5g ± 0.8g for Abcc5−/− females and 3.6g ± 0.8g for Abcc5−/− males (Fig. 1A and 1B). Weekly EchoMRI results showed that Abcc5−/− mice had proportionally less fat (expressed as a fraction of total body mass, Fig. 1C and 1D) and therefore more lean mass (expressed as a fraction of total body mass, Fig. 1E and 1F) with changes more pronounced in female mice than males. Differences in weight were statistically significant from 5 weeks of age and for all weeks onwards, analysed by a Student’s t-test for wild-type compared to Abcc5−/− mice for that week (Fig 1A and B). Changes in body composition over time shown in Figs 1A–F between wild-type and Abcc5−/− mice were analysed by two-way ANOVA with a Bonferroni post-hoc test and significance values are indicated by asterisks shown at the end of the two curves. Notably, mice were of equal weight at weaning. DEXA scans performed at week 14 showed no difference in bone mineral density (BMD) or bone mineral content (BMC) and X-rays confirmed that there were no changes in femur length, showing that growth is not stunted in Abcc5−/− mice (data not shown). Both female and male Abcc5−/− mice had significantly lower masses of white adipose tissues, including periovarian white adipose tissue (periWAT) depots in females and epididymal white adipose tissue (epiWAT) depots in male mice (Fig. 2A, B and F and Fig. 3A, B and F). Interscapular brown adipose tissue (iBAT) mass was significantly reduced in Abcc5−/− male mice (Fig. 3C, D and G) and a similar trend was observed in iBAT of Abcc5−/− female mice (Fig. 2C, D and G). Total fat mass in grams was decreased for both genders (Fig. 2E and 3E). Cholesterol and triglyceride plasma profiles showed decreased levels of total cholesterol, LDL, glycerol and triglycerides in both female and male Abcc5−/− mice, which would suggest that Abcc5−/− mice did not have dyslipidaemia (Fig. 2H and Fig. 3H).
Figure 1. Fat mass and body composition of Abcc5−/− (ko) mice and wild-type (wt) littermate controls.
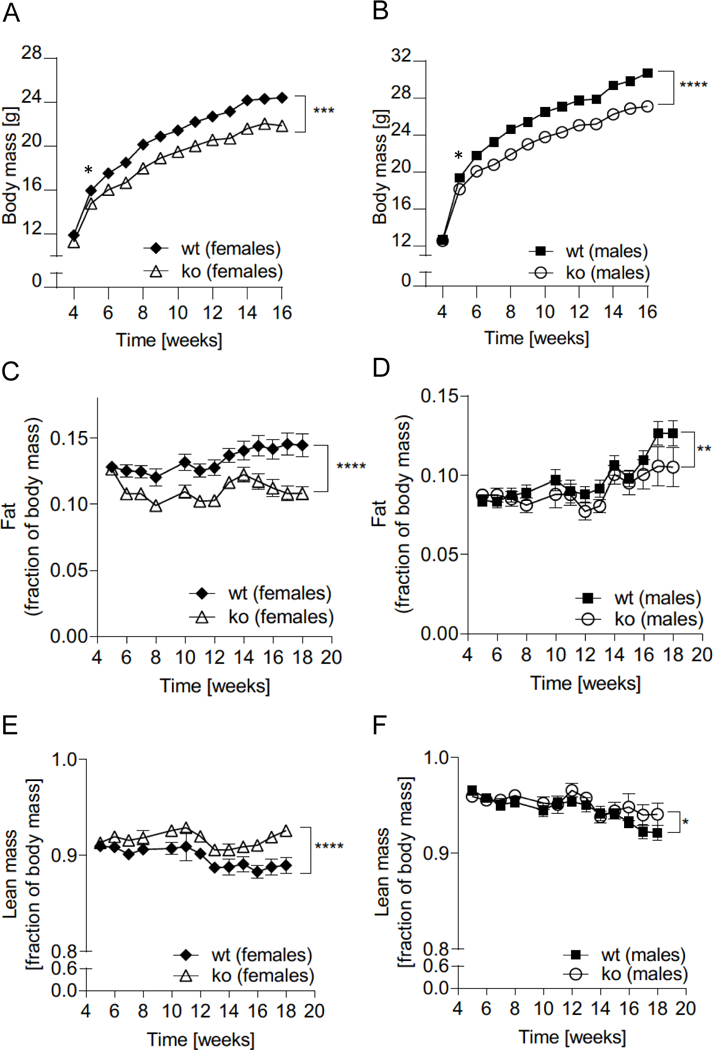
(A) Female and (B) male mice were weighed weekly from weaning over a period of 16 weeks. Data shown is for two separate co-horts for male:female:wt:Abcc5−/−=30:30:30:29. (C,D) Fat mass and (E,F) lean mass were recorded by EchoMRI for male:female:wt:Abcc5−/−=15:15:15:15 from 4 weeks to 18 weeks. Each data point represents mean±SEM. Changes in body mass, fat and lean mass over time for Abcc5−/− vs wt was analysed by two-way ANOVA with a Bonferroni post-hoc test. *P<0.05 **P≤0.01, ***P≤0.001, ****P≤0.0001. Differences in body mass between Abcc5−/− and wt mice (both female and male) were statistically significant from week 5 and for all weeks onwards when analysed by a Welch’s unequal variances t-test for that week, *P<0.05. Data for C-F is presented as a fraction of whole body mass measured at the same time point. Error bars smaller than the symbols are not visible on the graph.
Figure 2. Female white adipose tissue (WAT) and brown adipose tissue (BAT) depots.
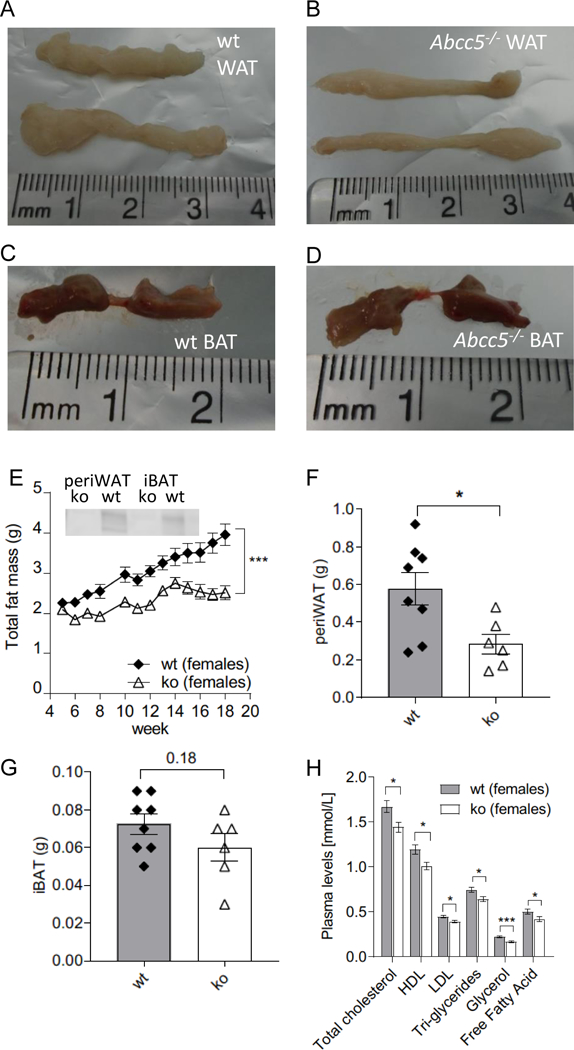
(A) Representative image of wild-type (wt) periovarian WAT (periWAT). (B) Representative image of Abcc5−/− (ko) periovarian WAT (periWAT). (C) Representative image of wt interscapular BAT (iBAT). (D) Representative image of Abcc5−/− interscapular BAT (iBAT). (E) Total fat mass. Each data point represents mean±SEM. Changes in total fat mass over time for Abcc5−/− (ko) vs wt were analysed by two-way ANOVA with a Bonferroni post-hoc test, ***P≤0.001. The insert shows Western blot analysis of ABCC5 protein expression in adipose tissue. Lanes 1 and 2, periovarian WAT (periWAT) from female Abcc5−/− mice and female wt mice respectively; lanes 4 and 5, intrascapular BAT (iBAT) from female Abcc5−/− and female wt mice respectively. (F) Mass of periWAT and (G) mass of iBAT, wt n=8, Abcc5−/− n=6 animals. (H) Lipid plasma levels for female Abcc5−/− mice (n=14) and female wt mice (n=15). Data shown as mean±SEM; Welch’s unequal variances t-test, *P<0.05, ***P<0.001.
Figure 3. Male brown adipose tissue (BAT) and white adipose tissue (WAT) depots.
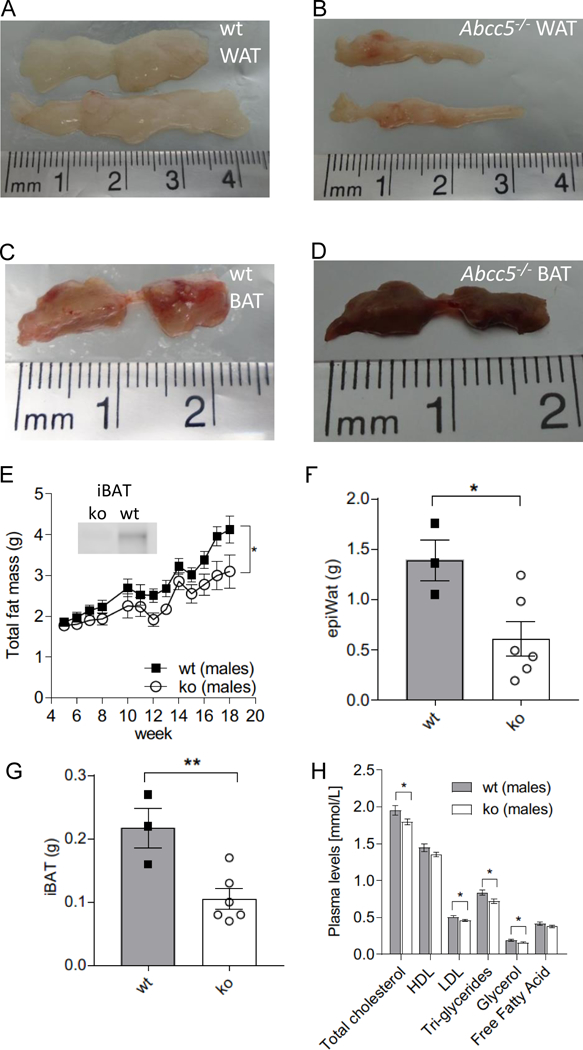
(A) Representative image of wild type (wt) epididymal WAT (epiWAT). (B) Representative image of Abcc5−/− (ko) epididymal WAT (epiWAT). (C) Representative image of wt interscapular BAT (iBAT). (D) Representative image of Abcc5−/− interscapular BAT (iBAT). (E) Total fat mass. Each data point represents mean±SEM. Changes in total fat mass over time for Abcc5−/− vs wt was analysed by two-way ANOVA with a Bonferroni post-hoc test, *P≤0.05. The insert shows Western blot analysis of ABCC5 protein expression in adipose tissue. Lanes 1 and 2, interscapular BAT (iBAT) for male Abcc5−/− and male wt mice respectively. (F) Mass of epiWAT and (G) mass of iBAT; wt, n=3, Abcc5−/−, n=6 animals. (H) Lipid plasma levels for male Abcc5−/− mice (n=15) and male wt mice (n=15). Data shown as mean±SEM; Welch’s unequal variances t-test, *P<0.05, **P<0.01.
Abcc5−/− mice are more active.
Indirect calorimetry analysis found that female Abcc5−/− mice had increased oxygen consumption (VO2), carbon dioxide release (VCO2) and energy expenditure (EE) in both dark and light cycles, while male mice did not (Fig. 4A–F). Both genders of Abcc5−/− mice were more active in both dark and light cycles, showing increased total activity, XT (the sum of ambulatory movement and fine movement) (Fig. 4G and 4H). No changes in respiratory exchange ratio (RER) were observed for either genders (data not shown), which would indicate that the energy source used by Abcc5−/− mice was not switched from carbohydrate (standard chow RER=0.9–1.0) to fat (RER=0.7). Abcc5−/− mice did not eat less, with female mice having slightly elevated food and water intake, while male Abcc5−/− mice showed no change (Fig. 5). The decreased fat depots in Abcc5−/− mice can therefore not be explained by hypophagia in Abcc5−/− mice.
Figure 4. Indirect calorimetry and metabolic cage analysis of Abcc5−/− (ko) mice.
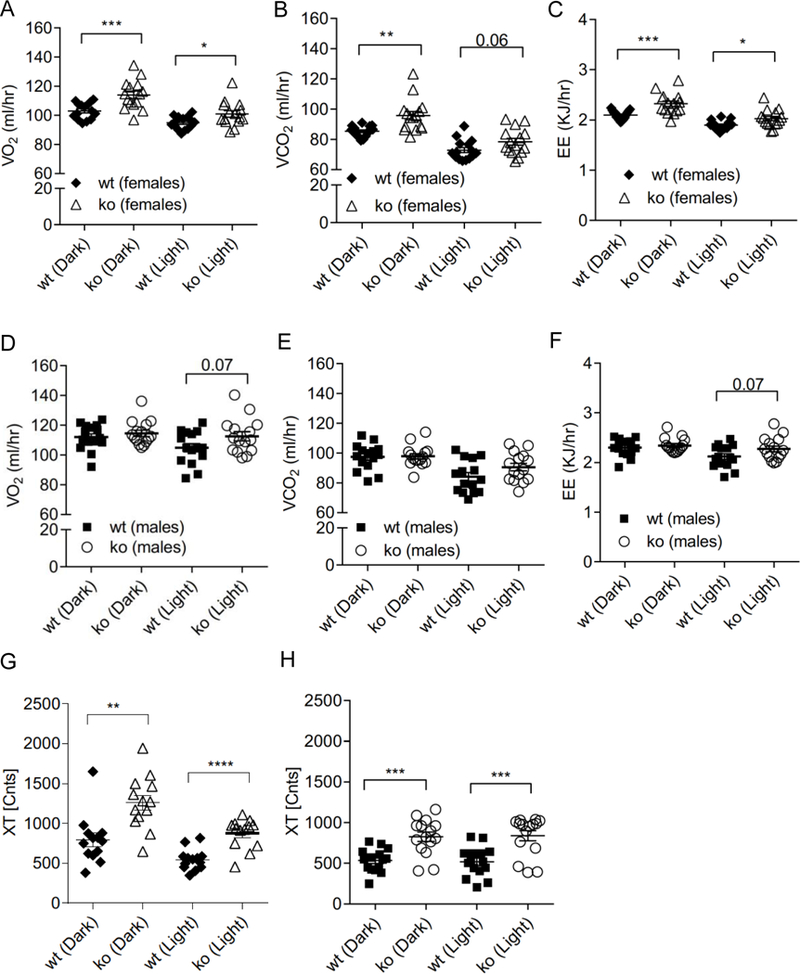
(A) Female oxygen consumption VO2. (B) Female carbon dioxide release VCO2. (C) Female energy expenditure. (D) Male oxygen consumption VO2. (E) Male carbon dioxide release VCO2. (F) Male energy expenditure. All data was ANCOVA adjusted for lean mass. Total activity (XT) is shown in (G) for females and (H) males. Abcc5−/− mice and wild type (wt) littermate controls (male:female:wt: Abcc5−/− =15:11:15:15) were individually housed for 24 hours in PhenoMaster cages at 12 weeks of age. The data shown in G and H is the average XT activity per hour plotted separately for light/dark cycle for each mouse. Error bars: mean±SEM; unpaired, two-tailed Student’s t-test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
Figure 5. Food and water intake.
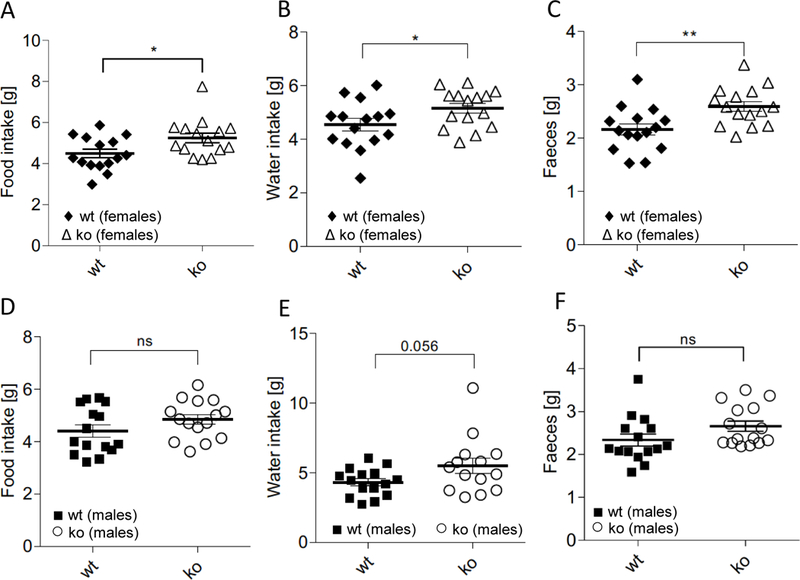
(A) Food intake females. (B) Water intake females. (C) Faecal production females. (D) Food intake males. (E) Water intake males. (F) Faecal production males. Data shown as mean±SEM, analysed using an unpaired, two tailed Student’s t-test (male:female:wt:Abcc5−/−=15:15:15:15), *P≤0.05,**P≤0.01.
Abcc5−/− mice are more insulin sensitive.
Female Abcc5−/− mice were able to lower plasma glucose more efficiently than wild-type littermate controls in response to an intraperitoneal (IP) insulin bolus following a 4h fast (Fig. 6A, and 6B), and the same trend was observed in male mice (Fig. 6C and 6D). Changes in plasma glucose in response to IP insulin over time for Abcc5−/− vs wild-type was analysed by two-way ANOVA with a Bonferroni post-hoc test (Fig. 6A and C). The area under the curve were analysed by unpaired, two-tailed Student’s t-test (Fig. 6B and D). An intraperitoneal glucose tolerance test (IPGTT) showed no phenotype dependent differences in response to glucose for either gender (Fig. 6E and 6F). However, a small but significant increase in plasma glucose levels were observed at 15min post glucose administration in female (Fig. 6E), but not male, Abcc5−/− mice (Fig. 6F), when analysed by multiple comparison two-way ANOVA with a Bonferroni post-hoc test (Fig. 6F). By contrast, no differences were observed in oral glucose tolerance in either sex (Fig. S2A and S2B). It is important to note that the increased insulin sensitivity observed in Abcc5−/− mice may therefore be secondary to the decreased adiposity of Abcc5−/− mice. By extension, it would appear that glucose-stimulated insulin secretion in Abcc5−/− mice is not greatly affected as plasma glucose levels following both IPGTT and OGTT show little or no change when compared to wild-type littermates. Future studies using a euglycemic, hyperinsulinemic clamp with stable isotopic glucose and water tracers will be required to delineate the basis of increased insulin sensitivity Abcc5−/− mice. Histological analysis of H&E (Haematoxylin and Eosin) stained gut, liver and pancreas samples showed no discernible changes in tissue morphology of Abcc5−/− mice when compared to wild-type littermates (data not shown).
Figure 6. Abcc5−/− (ko) mice show increased insulin sensitivity.
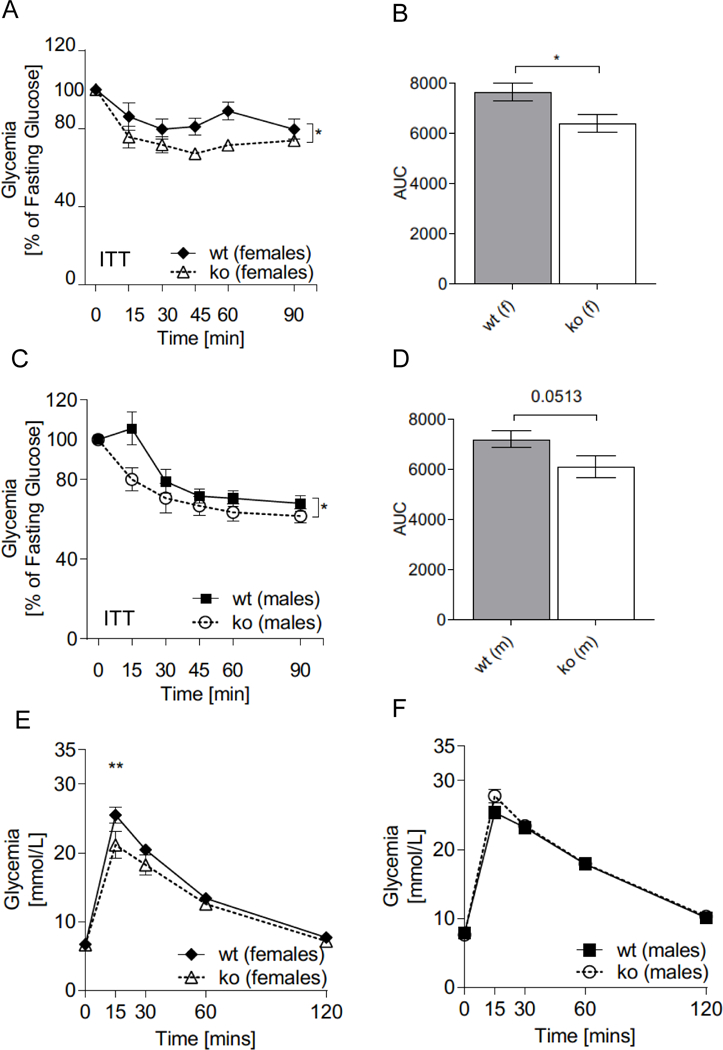
Insulin Tolerance Test (ITT) for (A,B) female and (C,D) male mice. (B,D) Calculated area under the curve (AUC). ITT was performed on mice aged 14 weeks, male:female:wt:Abcc5−/−=15:15:15:14. Data shown as mean±SEM. Changes in plasma glucose in response to IP insulin over time for Abcc5−/− vs wild-type (wt) was analysed by two-way ANOVA with a Bonferroni post-hoc test. Area under the curve were analysed by unpaired, two-tailed Student’s t-test, *P<0.05. (E) Intraperitoneal glucose tolerance tests (IPGTT) on 13 week old female and (F) male mice (male:female:wt:Abcc5−/−=15:15:15:14). Data shown as mean±SEM. Changes in plasma glucose in response to IP glucose over time for Abcc5−/− vs wt was analysed by multiple comparison two-way ANOVA with a Bonferroni post-hoc test, **P<0.01.
Abcc5−/− mice have raised plasma GLP-1 levels.
Circulating plasma levels of total GLP-1 measured 5min following an oral glucose gavage were increased by 60% in Abcc5−/− mice compared to wild-type littermate controls (Fig. 7A). In our hands, we were unable to measure baseline GLP-1 levels from plasma from mice fasted overnight, and only post-prandial GLP-1 levels could be detected. To determine the source of increased GLP-1 detected in the plasma, ex-vivo gut crypt primary cell cultures were grown from Abcc5−/− mice small intestine and analysed for GLP-1 secretion at rest and in the presence of glucose. Recapitulating the plasma results, Abcc5−/− mice gut primary cells secreted more active GLP-1 in both the presence and absence of glucose when compared to wild-type (Fig. 7B).
Figure 7. GLP-1 exocytosis is dependent on ABCC5 protein levels.
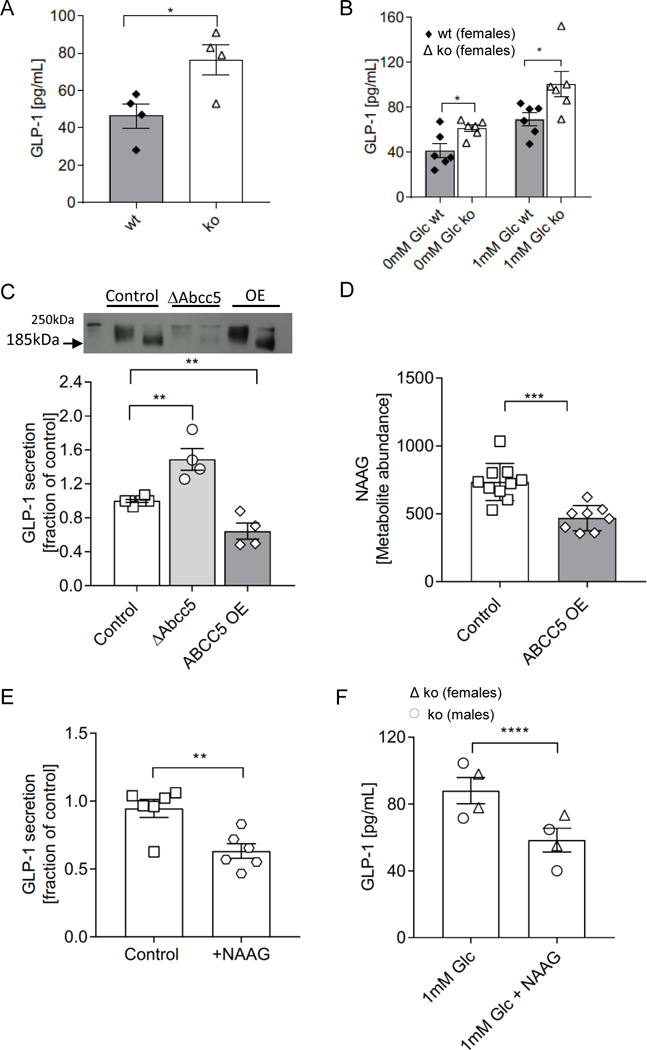
(A) Abcc5−/− (ko) mice (n=4, female, filled bar) had increased circulating levels of total plasma GLP-1 when compared to wild-type (wt) littermates (n=4, female, open bar) at t=5min following a fasted glucose challenge. (B) Active GLP-1 secretion from ex vivo gut crypt primary cell cultures was increased in Abcc5−/− mice (n=6) compared to wt littermates (n=6). (C) Total GLP-1 secretion from GLUTag cells following a 2h incubation in 5.6mM glucose (open squares, n=6) show increased GLP-1 release from Abcc5-siRNA treated GLUTag cells (open circles, n=6), while overexpression (OE) of ABCC5 protein attenuated GLP-1 release (open diamonds, n=4). Data presented as a fraction of GLP-1 secreted in 5.6mM (control) glucose. Data for A-C are shown as mean±SEM, and were analysed by unpaired Welch’s unequal variances t-test, *P≤0.05,**P≤0.01. The insert is a Western blot analysis of ABCC5 protein expression in wt GLUTag cells (lane 1, unheated sample; lane 2, heated sample), siRNA knockdown of Abcc5 gene expression (lanes 3, unheated sample; lane 4, heated sample) and recombinant overexpression of ABCC5 protein (lanes 5, unheated sample; lane 6, heated sample). ABCC5 typically migrates on a SDS-PAGE gel at a molecular weight of 185kDa in heated samples and at a higher molecular weight of about 200kDa in unheated samples. (D) ABCC5 overexpression (OE, open diamonds) decrease levels of NAAG in GLUTag cells when compared to control (open squares); control, n=10; ABCC5 OE, n=8. (E) Exogenous NAAG inhibits secretion of GLP-1 from GLUTag cells (open hexagons, n=6) when compared to control (open squares, n=6) in the presence of 1mM glucose. Data for D and E is shown as mean±SEM, and were analysed by an unpaired Student’s two tailed t-test; **P≤0.01, ***P≤0.001. (F) Exogenous NAAG inhibits secretion of GLP-1 from ex vivo gut crypt primary small intestine cultures of Abcc5−/− mice (n=4), ****P≤0.0001; unpaired Welch’s unequal variances t-test; Error bars: mean±SEM.
Abcc5 is expressed in mouse enteroendocrine cells.
Transcriptional profiling of the enteroendocrine cells of mouse duodenum, ileum and colon showed increased gene expression of Abcc5 in the enteroendocrine cells of the duodenum, and in preproglucagon expressing L-cells of the ileum when compared to non-endocrine cells (Table 1). GLUTag cells, a model L-cell line, expressed levels of Abcc5 mRNA similar to that observed in the enteroendocrine cells of the duodenum and ileum (Table 1). Interestingly, low resolution fluorescent microscopy images would indicate that ABCC5 is not expressed in the membranes of enteroendocrine cells and protein expression is shown to be intracellular in both GLUTag cells (Fig. S3A) and gut L-cells from GLUVenus mice (Fig. S3B).
Table 1. Expression of Abcc5 in mouse enteroendocrine cells (EEC).
Transcriptional profiling of Abcc5 expression in different regions of mouse GI tract and GLUTag cells, expressed in FPKM (Fragments per kilobase per million read). Fold enrichment (expressed as log2) and confidence of the enrichment (padj) is determined on normalised data using a DESeq model.
| Abcc5 | |||
|---|---|---|---|
| FPKM (gut endocrine) | FPKM (non- endocrine) |
log2FC positive / negative (padj) |
|
| duodenum_EEC | 9.739 | 1.903 | 2.050 (0.001) |
| ileum_EEC | 8.851 | 3.389 | 0.884 (0.414) |
| colon_EEC | 3.676 | 2.932 | 0.809 (0.640) |
| duodenum_L-cells | 7.939 | 3.350 | 1.525 (0.228) |
| ileum_L-cells | 12.353 | 2.039 | 1.928 (0.019) |
| colon_L-cells | 4.456 | 4.800 | 1.357 (0.116) |
| GLUTag cells | 6.491 | ||
GLP-1 exocytosis from gut enteroendocrine cells is inversely dependent on ABCC5 protein expression levels.
In order to test a direct link between ABCC5 protein expression and GLP-1 secretion, we used a well characterized model L-cell line, GLUTag cells, which secrete GLP-1 in response to stimulation by nutrients (23, 24). siRNA knock-down of Abcc5 gene expression in GLUTag cells resulted in a ∼60% increase in GLP-1 release (Fig. 7C), while recombinant overexpression of ABCC5 attenuated GLP-1 release below the exocytosis levels observed in the untreated control. ABCC5 protein expression levels in GLUTag cells used for GLP-1 secretion assays was confirmed by Western blot (Fig. 7D). Western blot analysis of wild type GLUTag cells showed robust ABCC5 protein expression (Fig. 7D, lanes 1 and 2) while siRNA knockdown of Abcc5 gene expression in GLUTag cells reduced ABCC5 protein expression levels substantially (Fig. 7D, lanes 3 and 4); recombinant overexpression of ABCC5 protein was also confirmed (Fig. 7D, lanes 5 and 6).
Using metabolomics, we identified a known ABCC5 substrate, N-acetylaspartylglutamate (NAAG) as an abundant glutamate metabolite in GLUTag cells. In order to confirm that the cellular levels of NAAG were also inversely related to ABCC5 expression in GLUTag cells, similar to that previously observed in HEK cells, ABCC5 was overexpressed in GLUTag cells and the levels of NAAG analysed by comparative metabolomics (18). The intracellular levels of NAAG were decreased in the ABCC5 overexpressing GLUTag cells when compared to sham transfected GLUTag cells (Fig. 7D). To investigate a potential role of this inhibitory neuropeptide in ABCC5-mediated regulation of gut hormone release, the effects of exogenous NAAG on GLP-1 levels were measured. The exogenous addition of 2 mM NAAG to both GLUTag cells and ex-vivo gut crypt primary cell cultures, generated from Abcc5−/− mice, inhibited the release of GLP-1 (Fig. 7E and 7F). Taken together this data would suggest that ABCC5 activity modulates GLP-1 release from gut endocrine cells through a NAAG dependent mechanism(s).
DISCUSSION
The most prominent metabolic phenotype of Abcc5−/− mice is a decrease in total levels of fat mass. Transcriptional profiling of human subcutaneous adipose tissue by Direk et al. showed high levels of ABCC5 gene expression and demonstrated that elevated expression of ABCC5 in subcutaneous adipose tissue confers an increased risk of developing type 2 diabetes with age in populations of disparate ancestry (1). The prevalence of type 2 diabetes was reported to be three times higher in subjects with high ABCC5 expression in in subcutaneous adipose tissue compared to those with low expression, and overexpression was most strongly associated with increased visceral white adipose tissue accumulation and reduced peripheral insulin sensitivity in nondiabetic individuals. Interestingly, the human overexpression phenotype is the opposite of our global Abcc5−/− mice, where both genders display decreased fat mass and increased insulin sensitivity. Adipose tissue is regulated by multiple endocrine and neurocrine inputs and Abcc5−/− mice showed decreases in both white adipose tissue (periovarian in females and epididymal in males) and interscapular brown adipose tissue in the absence of hypophagia i.e. Abcc5−/− mice did not eat less. Abcc5−/− mice were not burning fat in response to adaptive thermogenesis as there was no difference between the RER of Abcc5−/− and wild-type littermates and no browning was observed in the white adipose deposits of Abcc5−/− mice (data now shown). Both male and female mice were more active overall, but only in the females was increased activity coupled to increased energy expenditure as reflected in increased VO2. Increased energy expenditure could therefore contribute to the decreased fat deposits observed for female mice. On the other hand, male mice appear to have increased activity and decreased fat mass in the absence of changes in energy consumption (VO2), food intake or adaptive thermogenesis. It has been previously shown that more-active mice do not expend more energy under standard laboratory conditions (i.e. below thermoneutraility)(25). The decreased fat deposits in male Abcc5−/− mice would therefore suggest an additional role for ABCC5 in adipocyte physiology.
In addition to decreased fat mass, raised circulating plasma levels of GLP-1 were also observed in Abcc5−/− mice. Raised plasma GLP-1 appears to be the result of increased GLP-1 release from gut endocrine cells as ex vivo primary gut crypt cultures from Abcc5−/− mice also showed increased GLP-1 release, both at rest and in response to stimulation by glucose. Furthermore, an inverse relationship between ABCC5 protein expression and GLP-1 release was also confirmed in a model L-cell line, GLUTag cells, where recombinant over-expression of ABCC5 supressed GLP-1 secretion. ABCC5 has previously been identified as an amino-acid conjugate transporter and exports both N-lactoyl-amino acids and glutamate-aspartate conjugates from stably transfected HEK cells (18, 26). Jansen et al. demonstrated the accumulation of 8 glutamate conjugates in the tissues of Abcc5−/− mice using untargeted metabolomics, some of which are inhibitory neurotransmitters (18). Notably, the most abundant metabolite N-acetylaspartyl-glutamate (NAAG) is a glutamate neurotransmission antagonist and NAAG action downregulates excitatory glutamatergic neurons (27, 28). Glutamate also stimulates GLP-1 release from gut endocrine cells and it has been previously shown that glutamate and GLP-1 is loaded into the same vesicles in intestinal L-cells; and exogenous glutamate administration stimulate GLP-1 release from GLUTag cells (24, 29).
Here we were able to duplicate the HEK cell work done by Jansen et al. in GLUTag cells and demonstrated that ABCC5 also acts as an NAAG exporter in enteroendocrine cells. Furthermore, exogenous addition of NAAG inhibits GLP-1 release from both GLUTag cells and primary gut crypts. Intriguingly, as native ABCC5 protein expression in both GLUTag cells and L-cells of the ileum appears to be intracellular, and ABCC5 is a NAAG exporter, a possible explanation could be that ABCC5 is involved in loading this neuropeptide into synaptic-like vesicles of enteroendocrine cells, which are released upon exocytosis. The transport of glutamate and neuropeptides into both distinct and overlapping vesicle pools is well described in neurons but little is known about how NAAG is loaded into the synaptic vesicles in the brain (30). Therefore, if ABCC5 transporter activity is indeed involved in the export of inhibitory neurotransmitters such as NAAG, loss of this transporter could lead to a general increase in glutamatergic activation, such as is observed in the increase in total activity of both male and female mice as well as increased GLP-1 release from enteroendocrine cells. However, it has been shown that when recombinantly overexpressed in vitro, in addition to glutamate metabolites, ABCC5 may also transport organic anions (such as 6-mercaptopurine and thioguanine), pyrimidine-based antivirals such as 2’−3’-dideoxynucleotides, folates, various cyclic nucleotides as well as N-lactoyl-amino acids. We therefore cannot exclude the possibility that any of these other substrates may also be involved in the regulation of GLP-1 secretion from L-cells (26, 31–36).
In the brain, ABCC5 protein expression has been localized to astrocytes of the subcortical white matter as well as to pyramidal neurons (37). Interestingly, a recent report attributed the weight loss observed following the administration of the GLP-1 receptor agonist liraglutide, to increased glutamatergic signalling in the brain (38). GLP-1R activation in Abcc5−/− mice could therefore be upregulated through both increased circulating levels of GLP-1 and increased glutamatergic signalling due to a loss of NAAG inhibition.
In summary, Abcc5−/− mice have a surprisingly complex metabolic phenotype and are lean, have increased circulating plasma levels of GLP-1 and are more insulin sensitive. Abcc5−/− mice is the opposite of the observed human overexpression phenotype which is associated with increased visceral fat, insulin resistance and a susceptibility to type 2 diabetes with age. This study confirmed an important role for ABCC5 in adipocyte physiology in mammals. Future studies using inducible, tissue specific Abcc5−/− mice housed at thermoneutrality are now needed to dissect the metabolic implications of ABCC5 protein loss in the gut, the brain, the pancreas and adipose tissues.
Supplementary Material
What is already known about this subject?
A previous GWAS study linked the overexpression of an ATP-binding cassette transporter, ABCC5, to an increased risk of developing type 2 diabetes with age.
ABCC5 overexpression in humans was shown to be associated with reduced peripheral insulin sensitivity in nondiabetic individuals.
ABCC5 overexpression in humans was also shown to be most strongly associated with increased visceral fat accumulation.
What does this study add?
Opposite to the human overexpression phenotype, Abcc5 knock-out mice have decreased levels of adipose tissue.
Abcc5 knock-out mice have increased levels of plasma GLP-1 and have enhanced secretion of GLP-1 from gut endocrine cells.
There is an inverse relationship between ABCC5 protein expression and GLP-1 release, as recombinant overexpression of ABCC5 in GLUTag cells results in decreased secretion of GLP-1.
ACKNOWLEDGEMENTS
HdW would like to thank Prof. Xueming Tang for helpful discussion of the document.
FUNDING:
The MRC Harwell Institute is a member of the International Mouse Phenotyping Consortium (IMPC) and has received funding from the National Institutes for Health for generating the Abcc5−/− mice (UM1HG006348). The research reported in this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. MC and HdW was supported by the John Fell Fund (151/076 and 142/054), the Physiological Society (444) and the BBSRC (BB/P020666/1). AV is funded by a Novo-Nordisk post-doctoral fellowship. RDC and LB are supported by the MRC (MC_U142661184). JC is funded by a Diabetes UK RD Lawrence Fellowship. Research in the Reimann/Gribble lab is supported by the Wellcome Trust (106262/Z/14/Z, 106263/Z/14/Z).
Footnotes
DISCLOSURE: The authors declared no conflict of interest.
REFERENCES
- 1.Direk K, Lau W, Small KS, Maniatis N, Andrew T. ABCC5 transporter is a novel type 2 diabetes susceptibility gene in European and African American populations. Ann Hum Genet. 2014;78(5):333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10(12):729–40. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56 e5. [DOI] [PubMed] [Google Scholar]
- 4.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Jacobsen SH, Hansen DL, Worm D, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55(7):1890–901. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen NB, Jacobsen SH, Dirksen C, Bojsen-Moller KN, Naver L, Hvolris L, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–31. [DOI] [PubMed] [Google Scholar]
- 6.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring). 2008;16(2):298–305. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22(7):1084–96. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27(4):740–56. [DOI] [PubMed] [Google Scholar]
- 9.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyffer F, Tampe R. ABC transporters in adaptive immunity. Biochim Biophys Acta. 2015;1850(3):449–60. [DOI] [PubMed] [Google Scholar]
- 11.Blees A, Januliene D, Hofmann T, Koller N, Schmidt C, Trowitzsch S, et al. Structure of the human MHC-I peptide-loading complex. Nature. 2017;551(7681):525–8. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Pesce E, Sheppard DN, Singh AK, Pedemonte N. Therapeutic approaches to CFTR dysfunction: From discovery to drug development. J Cyst Fibros. 2018;17(2S):S14–S21. [DOI] [PubMed] [Google Scholar]
- 13.de Wet H, Proks P. Molecular action of sulphonylureas on KATP channels: a real partnership between drugs and nucleotides. Biochem Soc Trans. 2015;43(5):901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin GM, Yoshioka C, Rex EA, Fay JF, Xie Q, Whorton MR, et al. Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proks P, de Wet H, Ashcroft FM. Molecular mechanism of sulphonylurea block of K(ATP) channels carrying mutations that impair ATP inhibition and cause neonatal diabetes. Diabetes. 2013;62(11):3909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korolnek T, Zhang J, Beardsley S, Scheffer GL, Hamza I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 2014;19(6):1008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shipp LE, Hill RZ, Moy GW, Gokirmak T, Hamdoun A. ABCC5 is required for cAMP-mediated hindgut invagination in sea urchin embryos. Development. 2015;142(20):3537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen RS, Mahakena S, de Haas M, Borst P, van de Wetering K. ATP-binding Cassette Subfamily C Member 5 (ABCC5) Functions as an Efflux Transporter of Glutamate Conjugates and Analogs. J Biol Chem. 2015;290(51):30429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann A, Thompson A, Robbins N, Blomkalns AL. Localization, identification, and excision of murine adipose depots. J Vis Exp. 2014(94). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Psichas A, Tolhurst G, Brighton CA, Gribble FM, Reimann F. Mixed Primary Cultures of Murine Small Intestine Intended for the Study of Gut Hormone Secretion and Live Cell Imaging of Enteroendocrine Cells. J Vis Exp. 2017(122). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pais R, Rievaj J, Larraufie P, Gribble F, Reimann F. Angiotensin II Type 1 Receptor-Dependent GLP-1 and PYY Secretion in Mice and Humans. Endocrinology. 2016;157(10):3821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riffelmacher T, Clarke A, Richter FC, Stranks A, Pandey S, Danielli S, et al. Autophagy-Dependent Generation of Free Fatty Acids Is Critical for Normal Neutrophil Differentiation. Immunity. 2017;47(3):466–80 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhre RE, Wewer Albrechtsen NJ, Deacon CF, Balk-Moller E, Rehfeld JF, Reimann F, et al. Peptide production and secretion in GLUTag, NCI-H716, and STC-1 cells: a comparison to native L-cells. J Mol Endocrinol. 2016;56(3):201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47(9):1592–601. [DOI] [PubMed] [Google Scholar]
- 25.Virtue S, Even P, Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metab. 2012;16(5):665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen RS, Addie R, Merkx R, Fish A, Mahakena S, Bleijerveld OB, et al. N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc Natl Acad Sci U S A. 2015;112(21):6601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puttfarcken PS, Handen JS, Montgomery DT, Coyle JT, Werling LL. N-acetyl-aspartylglutamate modulation of N-methyl-D-aspartate-stimulated [3H]norepinephrine release from rat hippocampal slices. J Pharmacol Exp Ther. 1993;266(2):796–803. [PubMed] [Google Scholar]
- 28.Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69(1):174–81. [DOI] [PubMed] [Google Scholar]
- 29.Uehara S, Jung SK, Morimoto R, Arioka S, Miyaji T, Juge N, et al. Vesicular storage and secretion of L-glutamate from glucagon-like peptide 1-secreting clonal intestinal L cells. J Neurochem. 2006;96(2):550–60. [DOI] [PubMed] [Google Scholar]
- 30.Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97(13):7476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63(5):1094–103. [DOI] [PubMed] [Google Scholar]
- 33.Wielinga P, Hooijberg JH, Gunnarsdottir S, Kathmann I, Reid G, Zelcer N, et al. The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res. 2005;65(10):4425–30. [DOI] [PubMed] [Google Scholar]
- 34.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275(39):30069–74. [DOI] [PubMed] [Google Scholar]
- 35.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278(20):17664–71. [DOI] [PubMed] [Google Scholar]
- 36.Laue S, Winterhoff M, Kaever V, van den Heuvel JJ, Russel FG, Seifert R. cCMP is a substrate for MRP5. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(9):893–5. [DOI] [PubMed] [Google Scholar]
- 37.Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129(2):349–60. [DOI] [PubMed] [Google Scholar]
- 38.Adams JM, Pei H, Sandoval DA, Seeley RJ, Chang RB, Liberles SD, et al. Liraglutide Modulates Appetite and Body Weight Through Glucagon-Like Peptide 1 Receptor-Expressing Glutamatergic Neurons. Diabetes. 2018;67(8):1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


