Abstract
Importance:
Anhedonia, the difficulty experiencing pleasure, is a symptom of multiple psychiatric conditions in young adults that is associated with poorer mental health and psychosocial function and abnormal ventral striatum (VS) reward processing. Aberrant neural reward circuitry function is well-documented in anhedonia and other psychiatric disorders. Longitudinal studies to identify potential biomarkers associated with a reduction in anhedonia are necessary for the development of novel treatment targets.
Objective:
The purpose of this study was to identify neural reward-processing predictors of improved psychiatric symptoms and psychosocial function in a naturalistic, observational context.
Design:
A longitudinal follow-up study after baseline functional magnetic resonance imaging.
Setting:
A research program at the University of Pittsburgh Medical Center.
Participants:
Participants were between the ages of 18–25 experiencing psychological distress.
Main Outcomes/Measures:
Participants were evaluated at baseline and six months. At baseline, participants underwent functional magnetic resonance imaging during a card guessing monetary reward task. Participants completed measures of affective symptoms and psychosocial function at each visit. Neural activation during reward prediction error (RPE), a measure of reward learning, was determined using SPM12. Regions with significant RPE activation were entered as predictors of future symptoms in multiple linear regression models.
Results:
52 young adults [42F/10M, 21.7±2.3yrs] completed the study. Greater RPE activation in the left VS predicted a decrease in anhedonia symptoms over six months (β=−−6.152, p=0.035). The decrease in anhedonia between baseline and six months mediated the relationship between left VS activation to RPE and improvement in life satisfaction between baseline and six months (c-path: β=0.245; p=0.010; c’ path: β=0.133; p=0.161; ab path: 95%CI: 0.026,0.262). Results were not impacted by psychotropic medication usage.
Conclusions/Relevance:
Greater left VS responsiveness to RPE may serve as a biomarker, or potential target for novel treatments to improve, the severity of anhedonia, overall mental health, and psychosocial function.
Introduction
Young adulthood is a vulnerable developmental period in which psychiatric disorders emerge, including mood and anxiety disorders1. Nearly one-fifth of young adults between ages 18 and 25 seek mental healthcare for symptoms related to depression, mood, and anxiety2. These symptoms have negative effects on psychosocial function, including life satisfaction, work performance, and interpersonal relationships3,4. Most people with clinical-level affective psychopathology experience remission within six months5. Yet, there are few predictors and no objective neural biomarkers of future illness course and functional outcomes to guide prognosis or treatment.
Anhedonia, the difficulty experiencing pleasure, is an early defining feature of the depression that characterizes several psychiatric disorders6 including major depressive disorder (MDD) and bipolar disorder (BD). Anhedonia is an important symptom to monitor, as it is associated with treatment response7 and poorer psychosocial function3,8. Identifying biomarkers that predict future reduction in anhedonia may provide targets for novel treatments for numerous psychiatric disorders or markers of treatment response. This is particularly important in young adulthood, when interventions can take advantage of the neuroplasticity during this period9 to reduce severity of, or even prevent, psychiatric disorders.
Given anhedonia’s definition, neural circuits underlying reward learning – learning where mood and behaviors are modified in response to rewards – are especially relevant for studies identifying biomarkers associated with anhedonia6. This circuitry includes the ventral striatum (VS), ventrolateral prefrontal cortex (vlPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and amygdala. The VS supports aspects of reward processing10–27 and encodes the discrepancy between expected reward and actual reward outcome21 (reward prediction error [RPE]), a measure of reward learning and motivation to obtain future rewards28. The left vlPFC links stimuli to reward outcomes29,30 and participates in decision-making to obtain immediate rewards31,32. This laterality may be due to the role of the left prefrontal cortex in approach-related behavior33. The OFC encodes the incentive salience of expected rewards16–20,25,27; and the rostral-dorsal ACC guides behavior in response to incentive salience of stimuli to obtain rewards11,16,18. The amygdala interacts with the VS during reward and punishment, playing a unique role in reward processing27,34. Studies of the neural circuitry of anhedonia implicate these regions, although they are primarily among individuals with MDD and show abnormal VS activation to reward anticipation and receipt 21,35–38. Lower VS activation to reward is related to lower positive affect and higher anhedonia35. The relationship between greater anhedonia severity and lower VS activation to reward is consistent irrespective of depression severity39, as typically-developing individuals exhibit a relationship between greater anhedonia and reduced VS activation to reward receipt40.
Despite evidence for altered activation in reward circuitry, particularly VS, in individuals with psychiatric disorders associated with anhedonia, and the impact of symptoms on psychosocial function, no studies have focused on identifying neural biomarkers predicting future psychosocial function and anhedonia in young adults. Such biomarkers are critical for understanding symptom remission, treatment response, and developing novel treatments that may improve clinical and psychosocial function. One recent study demonstrated that adolescents with low VS activation to reward receipt were more likely to develop subthreshold depressive symptoms or meet full criteria for MDD over time41. It remains unclear, however, as to which psychiatric symptoms (including anhedonia) are specifically related to alterations in neural reward response, at which phase of reward processing these abnormalities occur, and how relationships between neural reward circuitry response and psychiatric symptoms relate to, or even predict, future psychosocial functioning in young adults.
In the current study, we recruited young adults (18–25 years) seeking mental healthcare due to psychologic distress (i.e. emotions negatively impacting level of functioning), irrespective of psychiatric diagnosis to examine neural predictors of future illness course and psychosocial functioning. Reward circuitry was examined using a monetary reward paradigm at baseline, with psychosocial functional and symptom trajectories examined over time. We hypothesized that response in neural regions underlying reward processing, including VS, would predict trajectories of future affective and anxiety symptoms. We specifically hypothesized that greater VS activation to RPE would be associated with a reduction in anhedonia severity over time. We further predicted that the reduction in anhedonia severity would be associated with improved psychosocial function. Lastly, we hypothesized that anhedonia severity reduction would mediate the relationship between neural reward response and psychosocial functioning.
Methods
Participants and Study Design
This study was approved by the University of Pittsburgh IRB. 52 individuals between ages 18–25 seeking mental healthcare for psychological distress were included in this prospective, longitudinal study. The goal was to recruit a young adult community sample during an age range when the majority of psychiatric illnesses first manifest and, as part of observing the typical course of depression without specific treatment intervention, to increase the likelihood for observing significant changes in clinical and psychosocial functioning over time42. Participants were recruited through community advertisement and student counseling centers in the Pittsburgh area and provided written informed consent. Individuals were right-handed and spoke fluent English. Of the 57 originally recruited, 3 were excluded due to incomplete data, one due to excessive task performance errors (20 errors, other participants <12), and one due to signal loss (>30%; see eMethods for full exclusion criteria).
Participants completed two study visits: 0 months (initial visit) and 6 months after the initial visit. 6 months was selected as the follow-up visit as this is the conventional timeframe for determining recovery from a depressive episode5 and thus appropriate for evaluating clinical and psychosocial outcomes. At the initial visit, participants underwent functional magnetic resonance imaging (fMRI) and completed clinician-rated and self-report assessments of depression, anxiety, anhedonia, and mania. Symptom measures were administered again at the follow-up visit. Participants were allowed to pursue treatment; psychotropic medication usage was collected at each visit and quantified per individual by computing the psychotropic medication load43 (see eMethods).
Affective and Psychosocial Function Measures
Participants’ self-reported affective symptoms were measured using the Mood and Anxiety Symptom Questionnaire44 – Anhedonic Depression subscale (MASQ-AD); MASQ – Anxious Arousal subscale (MASQ-AA); and the Snaith Hamilton Pleasure Scale45(SHAPS). Participants completed clinician rating scales: Hamilton Rating Scale for Depression46 (HRSD); Hamilton Anxiety Rating Scale47 (HAMA); and the Young Mania Rating Scale48 (YMRS). The Range of Impaired Functioning Tool49 (LIFE-RIFT) assessed psychosocial function across four domains (work, recreation, interpersonal relationships, and global satisfaction), with higher scores indicating greater functional impairment.
Monetary Reward fMRI Task
Neural activation during reward processing was evaluated using an adapted event-related card-guessing task50,51 that included win, loss, mixed, and neutral trials (see eFigure1). The primary outcome, reward prediction error (RPE), was determined as the difference in expected versus actual reward outcome. See eMethods for task description, MRI acquisition parameters, and preprocessing.
Data Analyses
For each participant, Statistical Parametric Mapping software (SPM12) was used to build a fixed-effect general linear model (GLM), using reward prediction error (RPE), reward expectancy (RE) and outcome expectancy (OE) regressors for first-level imaging analyses (see eMethods).
Functional connectivity maps were generated using generalized psychophysiological interaction (gPPI) using a priori reward regions previously shown to differentiate mood disordered from healthy individuals52,53 as seed regions: left vlPFC (Brodmann area [BA47]) and rostral-dorsal ACC (BA32) as defined by the Wake Forest University PickAtlas, and VS as defined by a prior meta-analysis of VS reward activation54 which we utilized as an a priori mask in our earlier studies50,53.
Individual contrast images were entered into group level SPM analyses. Age, gender, parental education, IQ, MRI scanner model, and change in psychotropic load during the study period were included as covariates in activation and connectivity models. Regions for activation analyses were constrained to a single mask comprising all reward regions of interest, defined by WFU PickAtlas: amygdala, rostral-dorsal ACC (BA32), OFC (BA11), and vlPFC (BA47); and VS as defined above53,54. Activation and connectivity maps were thresholded at a voxel pFWE<0.05. The BOLD response for individual regions with significant activation and connectivity within the reward mask in second-level analyses was extracted using Marsbar (http://marsbar.sourceforge.net/).
Multiple linear regression models, implemented in SPSSv23, tested whether baseline reward region activation and connectivity predicted changes in affective symptoms over six months. Change in symptoms was calculated as the difference between scores at baseline and follow up visits. Two separate multivariate linear regression models were run: one for self-reported affective symptoms (MASQ-AD, MASQ-AA, SHAPS) and another for clinician-rated affective symptoms (HRSD, HAMA, YMRS). Models were run separately, as type of rating scales contributes uniquely to symptom severity55. For each model, affective symptom changes were entered as dependent variables and the five neural regions with significant reward activation/connectivity (see Results below) were entered as independent variables. Pearson correlations were used to test the relationship between predictor variables (see eResults).
Mediation analyses were performed using the Preacher and Hayes bootstrapped mediation model implemented using the PROCESS macro in SPSS to examine whether changes in affective symptoms associated with reward circuitry response predicted domains of psychosocial function56. Activation in, and regions with connectivity to, regions of interest were entered as independent variables, with one independent variable per model. 6-month changes in affective symptoms were entered as mediators and 6-month change in psychosocial function domains were entered as dependent variables. All models, including mediation models, were corrected for multiple comparisons at p<0.05 using a Bonferroni correction.
Results
Participants
52 participants completed baseline and 6-month visits (Table 1). 39 participants met criteria for a DSM diagnosis (see eResults). Affective symptoms improved between baseline and follow up (see eTable1). 11 (29%) participants were started on psychotropic medication between baseline and follow-up (see eResults).
Table 1.
Baseline Participant Demographics by Gender
| N | Mean ± SD | ||
|---|---|---|---|
| Age (yrs) | 21.40 ± 2.25 | ||
| Gender | Female | 42 | |
| Male | 10 | ||
| IQ | 106.88 ± 7.84 | ||
| Race | White | 21 | |
| Black / African American | 8 | ||
| Asian | 10 | ||
| More than one race | 3 | ||
| Parental Education | Some high school | 1 | |
| High school / GED | 10 | ||
| Some college | 27 | ||
| Technical school | 2 | ||
| College degree | 12 | ||
| Current DSM Diagnosis | No current disorder | 13 | |
| Depressive disorder | 13 | ||
| Anxiety disorder | 26 | ||
| Externalizing disorder | 7 | ||
| Trauma-related disorder | 4 | ||
| Sleep disorder | 8 | ||
| Somatoform disorder | 3 | ||
| Adjustment disorder | 2 | ||
| Baseline Psychotropic Load | 0.17 ± 0.37 | ||
| Clinician Rated Affective Symptoms | Anxiety (HAMA) | 12.71 ± 6.66 | |
| Depression (HRSD) | 15.62 ± 6.77 | ||
| Mania (YMRS) | 2.77 ± 1.94 | ||
| Self-Reported Affective Symptoms | Anhedonic Depression (MASQ-AD) | 3.45 ± 0.68 | |
| Anxious Arousal (MASQ-AA) | 1.73 ± 0.69 | ||
| Anhedonia (SHAPS) | 27.40 ± 7.30 | ||
Activation in ROIs during RPE
Left and right VS, left and right rostral-dorsal ACC, and left amygdala were significantly activated to RPE within the reward mask (Table 2, Figure 1A). Whole-brain activation mirrored mask activation, where left and right VS/amygdala were activated as large clusters along with ACC, the inferior parietal lobule and middle cingulate cortex (see eTable2). No ROIs were activated significantly to RE and OE (see eTable2). No whole brain regions showed significant connectivity with seed regions.
Table 2.
Neural activation to reward prediction error (RPE). Thresholded at pFWE<0.05.
| Region | Hemisphere | Voxel pFWE | Voxels | T-score | x | y | z |
|---|---|---|---|---|---|---|---|
| Ventral Striatum | R | <0.001 | 129 | 6.97 | 18 | 12 | −10 |
| L | <0.001 | 42 | 6.27 | −10 | 14 | −4 | |
| Amygdala | L | 0.035 | 24 | 4.22 | −26 | 2 | −18 |
| Anterior Cingulate Cortex | R | 0.021 | 158 | 4.39 | 4 | 46 | 2 |
| L | 0.008 | 89 | 4.75 | −4 | 48 | −4 | |
Figure 1.
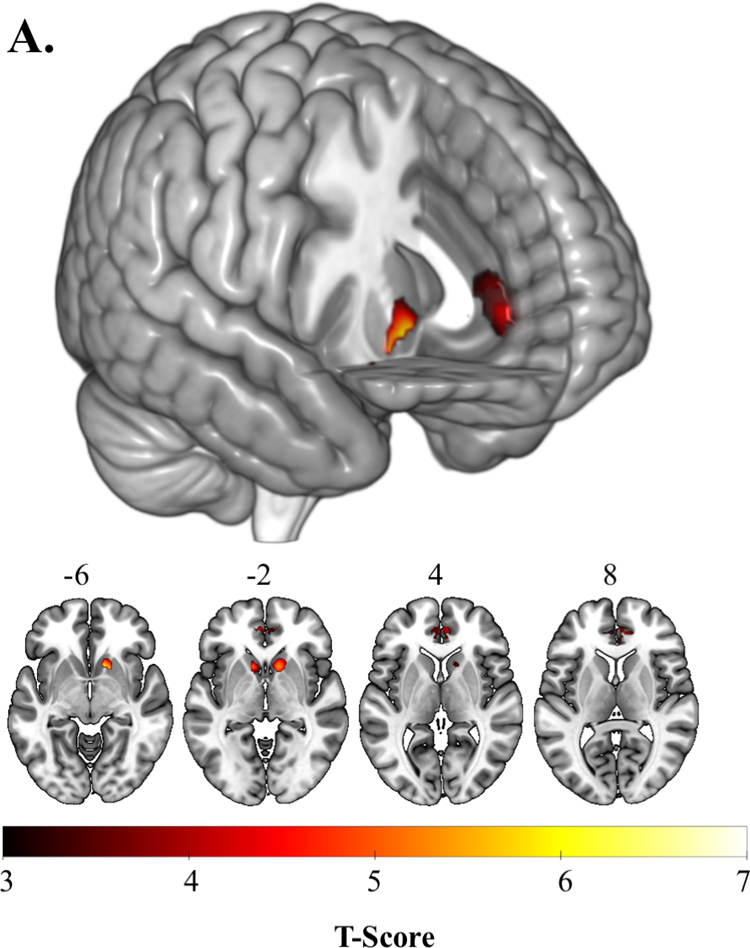
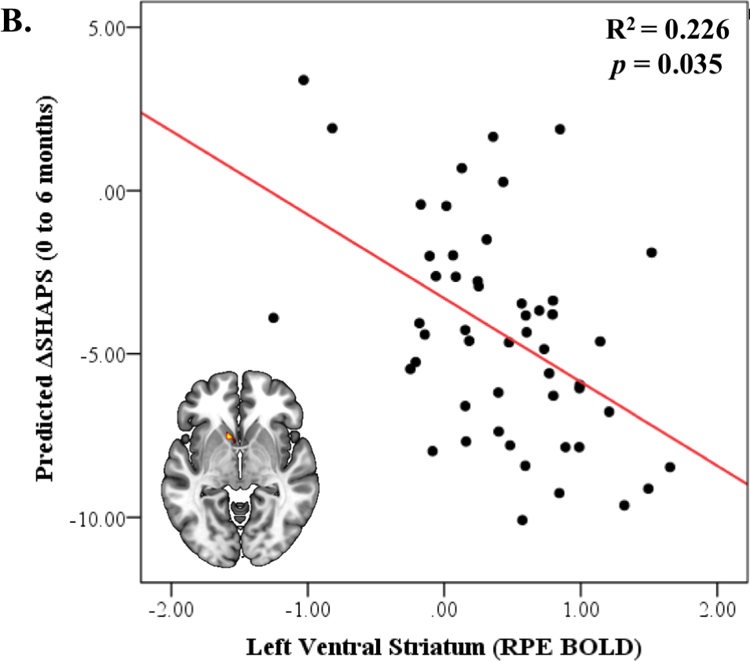
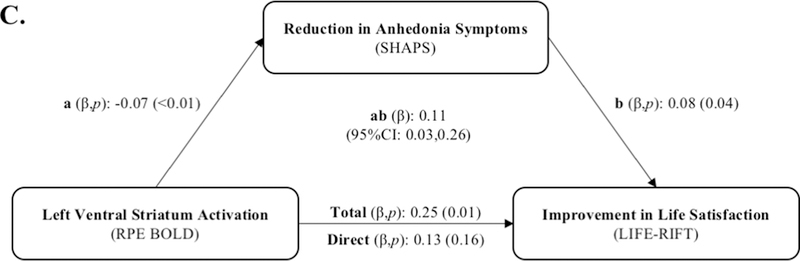
A. Activation to Reward Prediction Error (RPE) in the ventral striatum, amygdala, and anterior cingulate cortex. B. Left ventral striatum activation to RPE predicts decrease in anhedonia (SHAPS) severity over 6 months. C. Reduction in anhedonia severity at 6 months mediates the relationship between left ventral striatal activation during RPE and 6-month improvement in life satisfaction.
Association of Neural Activation to RPE with Improvement in Affective Symptoms
In a multiple linear regression model with multiple comparisons correction, left VS activation to RPE was negatively associated with change in self-reported anhedonia symptoms over 6 months (β=−6.152, p=0.035), where individuals with greater left VS activation demonstrated greater improvement in SHAPS (Figure 1B; Table 3). This association remained significant even after controlling for baseline SHAPS (β=−5.338, p=0.044). Right VS, left amygdala, left and right rostral-dorsal ACC activation to RPE did not predict self-reported affective symptoms. The multiple linear regression model predicting the 6-month change in clinician-reported affective symptoms based on neural activation to RPE was not significant (see eTable3). Psychotropic medication use did not moderate these results and including diagnosis in analyses did not change their significance (see eResults).
Table 3.
Association of neural activation to RPE with change in self-reported affective symptoms between baseline and 6 months
| 95% CI | |||||
|---|---|---|---|---|---|
| Affective Symptoms | B | p-value | LL | UL | |
| Anhedonic Depression (MASQ-AD) | Left Ventral Striatum | −0.079 | 0.799 | −0.792 | 0.545 |
| Left Amygdala | −0.360 | 0.093 | −0.782 | 0.062 | |
| Left ACC | −0.231 | 0.414 | −0.795 | 0.333 | |
| Right Ventral Striatum | 0.225 | 0.420 | −0.332 | 0.783 | |
| Right ACC | 0.398 | 0.227 | −0.257 | 1.054 | |
| Anxious Arousal (MASQ-AA) | Left Ventral Striatum | −0.029 | 0.894 | −0.466 | 0.409 |
| Left Amygdala | −0.058 | 0.693 | −0.361 | 0.238 | |
| Left ACC | −0.120 | 0.544 | −0.521 | 0.275 | |
| Right Ventral Striatum | 0.025 | 0.897 | −0.365 | 0.416 | |
| Right ACC | 0.018 | 0.937 | −0.456 | 0.478 | |
| Anhedonia (SHAPS) | Left Ventral Striatum | −6.152 | 0.036 | −11.870 | −0.433 |
| Left Amygdala | −1.451 | 0.454 | −5.318 | 2.416 | |
| Left ACC | −3.485 | 0.181 | −8.649 | 1.679 | |
| Right Ventral Striatum | 4.790 | 0.065 | −0.319 | 9.899 | |
| Right ACC | 4.129 | 0.173 | −1.876 | 10.134 | |
MASQ-AA, Mood and Anxiety Symptom Questionnaire – Anxious Arousal; MASQ-AD, Mood and Anxiety Symptom Questionnaire – Anhedonic Depression; SHAPS, Snaith Hamilton Pleasure Scale
6-month Improvement in Anhedonia Mediates the Association Between VS Activation to RPE and 6-month Improvement in Psychosocial Function
After correction for multiple comparisons using a Bonferroni correction, change in SHAPS between baseline and follow-up mediated the association between left VS activation to RPE and baseline-6-month change in LIFE-RIFT Satisfaction (Figure 1C, Table 4). Specifically, the total extent of the relationship between left VS activation to RPE and improvement in LIFE-RIFT Satisfaction over 6 months (c-path: β=0.245; p=0.010) was accounted for by the change in anhedonia severity over 6 months (ab path: 95%CI:0.026,0.262); after accounting for the reduction in anhedonia, this relationship was no longer significant (c’ path: β=0.133; p=0.161). Including psychotropic medication as a covariate did not change the significance of the results (see eTable4), and psychotropic medication use did not moderate these results (see eResults). Including diagnosis in analyses similarly did not change the significance of the results (see eResults).
Table 4.
6-month change in anhedonia symptoms mediates the relationship between left VS activation to RPE and improved life satisfaction
| Mediation Model [β(p)] | Bootstrapping bias-corrected 95% CI | |||||
|---|---|---|---|---|---|---|
| LIFE-RIFT | Direct (c’) | Total (c) | Effect (PM) | SE | Lower Level CI | Upper Level CI |
| Total | −0.013 (0.657) | 0.017 (0.598) | 1.779 | 0.019 | 0.003 | 0.081 |
| Work | −0.018 (0.807) | 0.020 (0.808) | 1.900 | 0.040 | −0.029 | 0.136 |
| Interpersonal Relationships | −0.032 (0.726) | 0.080 (0.408) | 1.401 | 0.060 | 0.023 | 0.264 |
| Satisfaction† | 0.133 (0.161) | 0.245 (0.010) | 0.456 | 0.060 | 0.026 | 0.262 |
| Recreation | −0.095 (0.143) | −0.113 (0.121) | 0.156 | 0.034 | −0.103 | 0.041 |
Coefficients in boldface denote significant mediation. CI = Confidence interval.
Significant at p<0.05 with Bonferroni correction
Discussion
This is the first prospective, longitudinal study to identify a transdiagnostic neural biomarker for improvement in psychiatric symptoms from a dimensional perspective and psychosocial function in a community sample of young adults. Neural reward regions including VS, rostral-dorsal ACC, and amygdala were significantly activated during RPE. Of these neural regions, greater left VS activation to RPE predicted improvement in self-reported anhedonia severity over 6 months, and this improvement mediated the relationship between left VS activation to RPE and improved life satisfaction. Activation to RPE in other reward regions did not predict 6-month change in self-reported affective and anxiety severity and change in clinician-rated psychiatric symptom severity was not predicted by activation in any reward circuitry regions.
Recent studies have identified potential neural biomarker predictors of psychiatric illness progression. Two independent studies examined the development of depression in healthy individuals. In one, lower bilateral VS activation to anticipated monetary reward in adolescents was associated with prospective development of MDD over two years41. The other reported left VS resting state functional connectivity predicted the onset of depressive symptoms after three years57. In our sample of young adults who already started experiencing psychologic distress, left VS activation to RPE predicted progression of anhedonia severity, but not overall depressive or anxiety severity. This may be due to the role of VS in encoding motivational aspects of reward. Phasic firing of dopaminergic neurons in the ventral tegmental area encode and transmit RPE signals to VS to facilitate goal-directed behavior58. The relationship between greater VS activation to RPE and improvement in anhedonia suggests individuals with greater VS activation to RPE may retain capacity for reward learning and motivation to obtain rewards. This may facilitate recovery from anhedonia symptoms over time. In contrast, lower VS activation to RPE suggests an impaired ability to learn from, and be motivated by, rewards, which may perpetuate anhedonia. Recovery from other symptoms, such as anxiety and depression, may involve a more distributed reward network beyond VS or be dependent on neural regions beyond reward circuitry; however, this is the first longitudinal study to identify a psychiatric neural biomarker of a dimensional construct (i.e. anhedonia) that may play a critical role in the development of numerous psychiatric disorders
One important question in determining potential biomarkers is whether a single measurement of VS activation is reliable. Animal models suggest RPE-related activation may be stable over time59,60, and one study in healthy children found that negative RPE encoding (e.g., the omission of a predicted reward) was stable across three years in the insula while positive RPE encoding (e.g. the presence of an unpredicted reward, as measured in the present study) was stable only across a period of months61. The authors hypothesized this may be due to several factors, including premature responses on trials and signal-to-noise ratio. Our finding that left VS activation to positive RPE predicts improvement in anhedonia symptoms over a period of months parallels these results and suggests left VS activation to positive RPE may be a potential biomarker for short-term progression of anhedonia severity.
In the present sample, left and right VS were not differentially activated to RPE, although only left VS activation to RPE predicted the trajectory of anhedonia severity. This is consistent with previous reports supporting a role for left VS in integrating information from emotion processing and reward regions57,62,63. While no reward regions showed significant connectivity with the VS to RPE in the present sample, the activation results nonetheless might have been influenced by the integration of signals from non-reward regions. Resting state connectivity analyses reveal lateralized patterns of connectivity between left and right VS, with left VS exhibiting greater connectivity with dorsomedial prefrontal cortex and the posterior cingulate gyrus64. Heightened connectivity of left VS with these default mode regions suggests a lateralization of internally-directed and self-regulatory processes that are known to be disrupted in depressive disorders6,57. These findings support the importance of examining the laterality of potential biomarker predictors of future clinical and psychosocial outcome measures.
Limited research has examined relationships between neural and psychosocial function, and no studies have examined neural predictors of future psychosocial function. One study found that self-reported anxiety mediated the relationship between amygdala and vlPFC activation and overall psychosocial function65. While it is not surprising that the reduction in anhedonia was associated with improved life satisfaction, given the relationship between anhedonia and decreased experience of pleasure, this is the first prospective study to identify a neural region associated with improved psychosocial function. This suggests that the left VS may be a particularly salient neural target for improving anhedonia severity and life satisfaction.
Strengths and Limitations
There was no significant activation to the other two main regressors, RE and OE, in the present study. While previous findings from the current sample indicated robust patterns of activation to RE53, this earlier study focused on individual differences in behavioral traits and associations with RE-related activation among healthy and psychologically-distressed individuals. By contrast, the present study examined patterns of neural reward activation that were common to young adults with psychological distress and examined how this pattern of neural activation predicted future symptom changes. While we did not find specific effects of medication, only 11 participants were taking psychotropic medication at follow-up, with variability in medication type, dosing, and duration. Our findings replicate the natural course of depression where symptoms partially remit over time even without treatment 66,67; yet, additional research is needed to determine how neural biomarkers may also predict recurrence and future severity of depression. One limitation is the absence of a 6-month scan, which could examine the specificity of the relationship between observed symptoms and left VS activation; however, this study’s purpose was to identify neural biomarkers at time of presentation in psychological distress that predict future symptoms and psychosocial function.
Conclusion
Our findings identify a reward circuitry predictor of anhedonia reduction, and a specific directional relationship between reduction in anhedonia severity and improved psychosocial function, in young adults experiencing psychological distress. This is the first longitudinal, prospective study to identify neural biomarker predictors of psychiatric symptom reduction and improved psychosocial function in young adulthood, a critical period of development when psychiatric symptoms typically emerge. Left VS activation to RPE predicts a reduction in anhedonia severity, and this reduction mediates the relationship between greater left VS activation and improvement in life satisfaction. Our findings suggest left VS activation to RPE can, in future studies, be used to monitor response to treatments for anhedonia, and that the left VS can ultimately be used as a target for novel interventions to facilitate anhedonia reduction and psychosocial function improvement in young adults.
Supplementary Material
Key Points.
Question:
Which neural reward regions predict improved psychiatric symptoms and psychosocial function in young adults?
Findings:
In this longitudinal neuroimaging study, reward activation in the left ventral striatum predicted improvement in anhedonia symptoms over 6 months. The reduction in anhedonia mediated the relationship between left ventral striatal reward activation and improvement in psychosocial function.
Meaning:
Left ventral striatum may be a plausible biomarker for novel treatments to improve psychiatric symptoms and psychosocial function.
Acknowledgments
This work was supported by the National Institute of Mental Health (R01MH100041 to MLP) and the Pittsburgh Foundation (to MLP).
Role of Funder/Sponsor Statement: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 2007;20(4):359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SAMHSA. Results from the 2006 National Survey on Drug Use and Health: National Findings Rockville, MD: 2007. [Google Scholar]
- 3.Rapaport MH, Clary C, Fayyad R, Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry 2005;162(6):1171–1178. [DOI] [PubMed] [Google Scholar]
- 4.Olatunji BO, Cisler JM, Tolin DF. Quality of life in the anxiety disorders: a meta-analytic review. Clin Psychol Rev 2007;27(5):572–581. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Depression in Adults: Recognition and Management NICE Guideline (CG90) 2009. [PubMed] [Google Scholar]
- 6.Nusslock R, Alloy LB. Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord 2017;216:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry 2012;51(4):404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guajardo VD, Souza BP, Henriques SG, et al. Loss of interest, depressed mood and impact on the quality of life: cross-sectional survey. BMC Public Health 2011;11:826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat 2013;9:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 2003;18(2):263–272. [DOI] [PubMed] [Google Scholar]
- 11.Rogers RD, Ramnani N, Mackay C, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry 2004;55(6):594–602. [DOI] [PubMed] [Google Scholar]
- 12.Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa Gambling Task. Neuroimage 2005;24(1):253–259. [DOI] [PubMed] [Google Scholar]
- 13.Ernst M, Dickstein DP, Munson S, et al. Reward-related processes in pediatric bipolar disorder: a pilot study. J Affect Disord 2004;82 Suppl 1:S89–S101. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt L, Clery-Melin ML, Lafargue G, et al. Get aroused and be stronger: emotional facilitation of physical effort in the human brain. J Neurosci 2009;29(30):9450–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage 2004;23(1):64–74. [DOI] [PubMed] [Google Scholar]
- 16.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron 2011;70(6):1054–1069. [DOI] [PubMed] [Google Scholar]
- 17.May JC, Delgado MR, Dahl RE, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry 2004;55(4):359–366. [DOI] [PubMed] [Google Scholar]
- 18.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci 2011;15(2):56–67. [DOI] [PubMed] [Google Scholar]
- 19.Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci 2003;23(1):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage 2004;23(3):777–786. [DOI] [PubMed] [Google Scholar]
- 21.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain 2008;131(Pt 8):2084–2093. [DOI] [PubMed] [Google Scholar]
- 22.Schultz W Getting formal with dopamine and reward. Neuron 2002;36(2):241–263. [DOI] [PubMed] [Google Scholar]
- 23.Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 2001;32(3):537–551. [DOI] [PubMed] [Google Scholar]
- 24.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 2001;30(2):619–639. [DOI] [PubMed] [Google Scholar]
- 25.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 2001;12(17):3683–3687. [DOI] [PubMed] [Google Scholar]
- 26.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 2000;84(6):3072–3077. [DOI] [PubMed] [Google Scholar]
- 27.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz W Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci 2016;17(3):183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SW, O’Doherty JP, Shimojo S. Neural computations mediating one-shot learning in the human brain. PLoS Biol 2015;13(4):e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boorman ED, Rajendran VG, O’Reilly JX, Behrens TE. Two Anatomically and Computationally Distinct Learning Signals Predict Changes to Stimulus-Outcome Associations in Hippocampus. Neuron 2016;89(6):1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith BJ, Monterosso JR, Wakslak CJ, Bechara A, Read SJ. A meta-analytical review of brain activity associated with intertemporal decisions: Evidence for an anterior-posterior tangibility axis. Neuroscience & Biobehavioral Reviews 2018;86:85–98. [DOI] [PubMed] [Google Scholar]
- 32.Hill PF, Yi R, Spreng RN, Diana RA. Neural congruence between intertemporal and interpersonal self-control: Evidence from delay and social discounting. NeuroImage 2017;162:186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson RJ, Shackman AJ, Maxwell JS. Asymmetries in face and brain related to emotion. Trends Cogn Sci 2004;8(9):389–391. [DOI] [PubMed] [Google Scholar]
- 34.Baxter MG, Murray EA. The amygdala and reward. Nature reviews neuroscience 2002;3(7):563. [DOI] [PubMed] [Google Scholar]
- 35.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry 2009;166(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 2010;67(5):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009;166(6):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keren H, O’Callaghan G, Vidal-Ribas P, et al. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. Am J Psychiatry 2018:appiajp201817101124. [DOI] [PMC free article] [PubMed]
- 39.Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord 2012;136(3):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 2009;46(1):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stringaris A, Vidal-Ribas Belil P, Artiges E, et al. The Brain’s Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. Am J Psychiatry 2015;172(12):1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessler RC, Amminger GP, Aguilar‐Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current opinion in psychiatry 2007;20(4):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry 2010;67(5):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 1991;100(3):316–336. [DOI] [PubMed] [Google Scholar]
- 45.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995;167(1):99–103. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton M A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton M The assessment of anxiety states by rating. British journal of medical psychology 1959;32(1):50–55. [DOI] [PubMed] [Google Scholar]
- 48.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 49.Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol Med 1999;29(4):869–878. [DOI] [PubMed] [Google Scholar]
- 50.Chase HW, Fournier JC, Bertocci MA, et al. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Transl Psychiatry 2017;7(4):e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckstrand KL, Hanford LC, Bertocci MA, et al. Trauma-associated anterior cingulate connectivity during reward learning predicts affective and anxiety states in young adults. Psychol Med 2018:1–10. [DOI] [PMC free article] [PubMed]
- 52.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry 2013;170(5):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord 2013;15(8):839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 2012;50(7):1252–1266. [DOI] [PubMed] [Google Scholar]
- 55.Uher R, Perlis RH, Placentino A, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety 2012;29(12):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression Based Approach New York, NY: The Guilford Press; 2013. [Google Scholar]
- 57.Pan PM, Sato JR, Salum GA, et al. Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disorder in a Longitudinal Community-Based Sample. Am J Psychiatry 2017;174(11):1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar P, Goer F, Murray L, et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 2018;43(7):1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keiflin R, Janak PH. Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron 2015;88(2):247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci 1998;1(4):304–309. [DOI] [PubMed] [Google Scholar]
- 61.Keren H, Chen G, Benson B, et al. Is the encoding of Reward Prediction Error reliable during development? Neuroimage 2018;178:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davey CG, Whittle S, Harrison BJ, et al. Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychol Med 2015;45(5):1001–1009. [DOI] [PubMed] [Google Scholar]
- 63.Connolly CG, Wu J, Ho TC, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry 2013;74(12):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang S, Hu S, Chao HH, Li CR. Hemispheric lateralization of resting-state functional connectivity of the ventral striatum: an exploratory study. Brain Struct Funct 2017;222(6):2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenberg T, Bertocci MA, Chase HW, et al. Mediation by anxiety of the relationship between amygdala activity during emotion processing and poor quality of life in young adults. Transl Psychiatry 2017;7(7):e1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med 1995;25(6):1171–1180. [DOI] [PubMed] [Google Scholar]
- 67.Ramana R, Paykel ES, Cooper Z, Hayhurst H, Saxty M, Surtees PG. Remission and relapse in major depression: a two-year prospective follow-up study. Psychol Med 1995;25(6):1161–1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


