FIG 1.
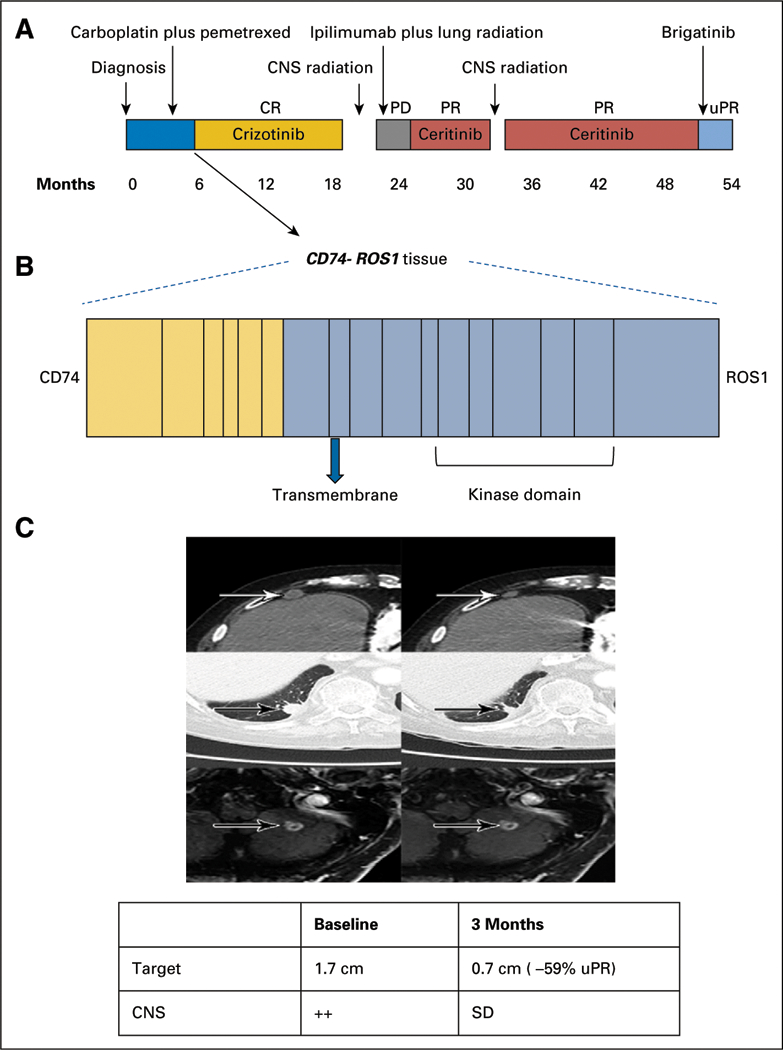
Clinical activity of brigatinib in crizotinib-and ceritinib-resistant ROS1-positive non–small-cell lung cancer. (A) The various treatments the patient received for metastatic ROS1-positive non–small-cell lung cancer, along with the duration of and best response to each treatment. (B) Fusion event: 5ˊ-CD74(x1–6 NM_004355)-3ˊ-ROS1(x33–43 NM_002944) breakpoints CD74 intron 6, ROS1 intron 32. (C) Computed tomography and magnetic resonance images of the patient’s right anterior diaphragmatic lymph node, right lower lobe nodule, and left cerebellar metastases before and at the indicated times after he initiated treatment with brigatinib. A radiologic unconfirmed partial response by Response Evaluation Criteria in Solid Tumors 1.1 was achieved after 3 months in the target lesion (right lower lobe nodule), with concurrent response in the right anterior diaphragmatic lymph node and stable cerebellar metastases. CR, complete response; PD, progressive disease; PR, partial response; uPR, unconfirmed partial re-sponse; SD, stable disease.
