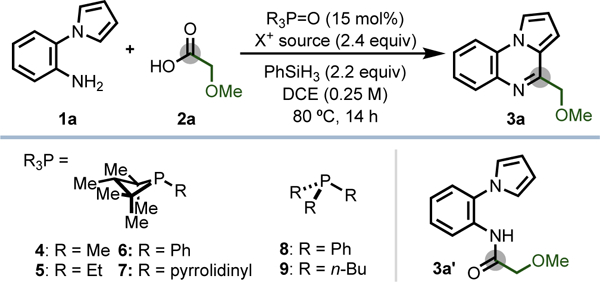
Table 1.
Discovery and Optimization of Phosphacatalytic Iterative Condensation/Annulation of Amine and Carboxylic Acid
| Entry | R3P=O | X+ source | Yield (%)a |
|---|---|---|---|
| 1 | 4•[O] | DEBM | 94 (84)b |
| 2 | 4•[O] | DEMBM | 90 |
| 3 | 4•[O] | NBS | 10 |
| 4 | 4•[O] | DECM | 0 |
| 5 | 4•[O] | CCl4 | 0 |
| 6 | 5•[O] | DEBM | 26 |
| 7 | 6•[O] | DEBM | 46 |
| 8 | 7•[O] | DEBM | 50 |
| 9 | 8•[O] or 9•[O] | DEBM | 0 |
| 10 | none | DEBM | 0 |
| 11 | 4•[O] | none | 0 |
| 12 | 4•[O], no PhSiH3 | DEBM | 0 |
| 13 | 4 | DEBM | 90 |
| 14 | [4•Br]Br | DEBM | 87 |
Yields determined through 1H NMR analysis with the aid of an internal standard. See Supporting Information for full synthetic details and yields of intermediate amide 3a’.
Isolated yield on 0.4 mmol scale. DCE = 1,2-dichloroethane; DEBM = diethyl bromomalonate; DEMBM = diethyl (methyl)bromomalonate; NBS = N-bromosuccinimide; DECM = diethyl chloromalonate.