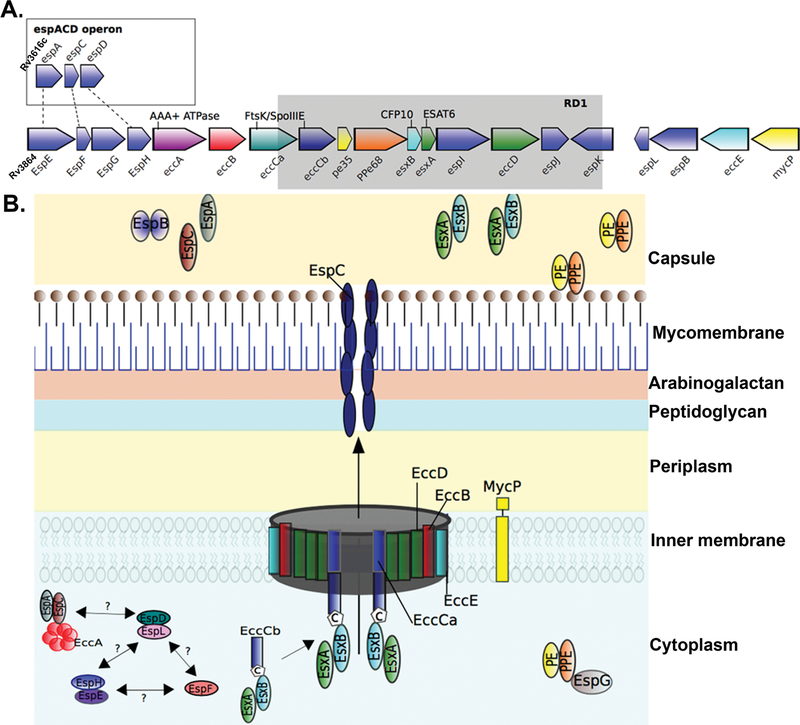
FIGURE 1.
Schematic of the ESX-1 secretion system. (A) Gene map of the esx-1 locus and the espACD operon in M. tuberculosis H37Rv. The esx-1 locus includes esx genes encoding the secreted effector proteins EsxA and EsxB alongside ecc genes encoding ESX-conserved proteins and esp genes encoding ESX secretion-associated proteins (108). The espACD operon is at a locus distinct from the esx-1 locus but shares sequence homology with espE, espF, and espH of esx-1 (dashed lines). The spontaneous deletion (Rv3871 to Rv3878) from esx-1 found in the vaccine strains of M. bovis BCG is known as region of difference 1 (RD1) and is indicated by the gray box. (B) Model of the ESX-1 secretion system in the mycobacterial cell envelope. In common with all ESX systems, the core structure of the ESX-1 secretion apparatus starts with the inner membrane-spanning conserved components EccB, EccC, EccD, and EccE (109). EccC is an ATP-driven translocase consisting of two subunits (a and b) that are assembled following EccB binding of target substrate, in this case, the heterodimer EsxAB, where EccCb interacts with the carboxyl-terminal signal sequence of EsxB (labeled “C”) (21, 110). EsxAB secretion is codependent on the secretion of EspC/EspA, which is also dependent on interaction with the cytosolic ATPase EccA (20, 111). EspC polymerizes during secretion, indicating a role for EccA and EspA as cytosolic chaperones (18), and forms a filamentous structure thought to provide a channel for secretion of ESX-1 substrates (18). Other important ESX-1 substrates include the PE and PPE families of proteins, which form heterodimers and are recruited by the putative cytosolic chaperone EspG to initiate interaction with the core complex of proteins within the inner membrane (112–114). EspB is also secreted by ESX-1 and forms a PE-PPE-like fold, containing a C-terminal domain that is processed by the MycP1 protease during secretion (26, 115). EspD to -F and EspH proteins are cytosolic and were recently shown to be stabilized by the cytosolic chaperone EspL (24–26).