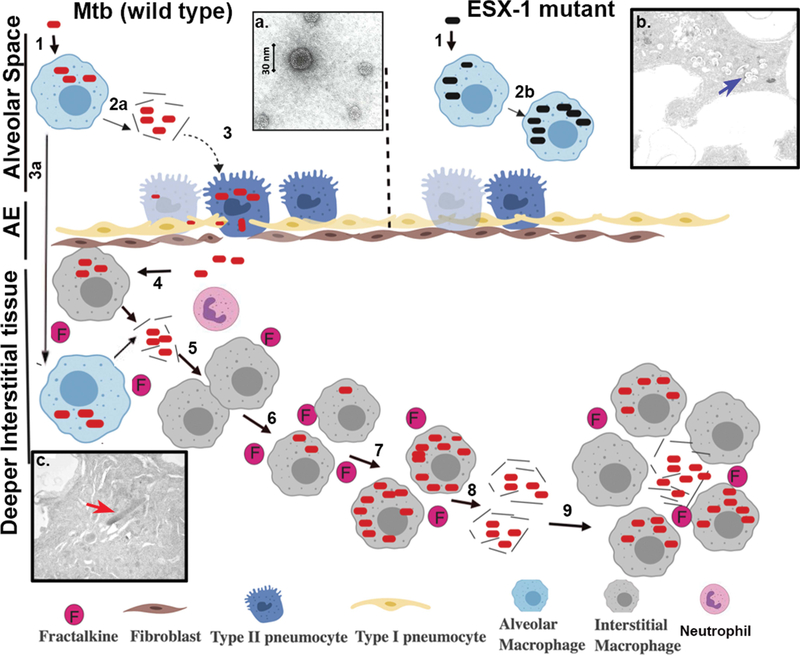
FIGURE 2.
ESX-1-related disease progression within the lung. Steps involved in progression of disease are represented with the numbers 1 to 9. (1) Infection of alveolar macrophages with M. tuberculosis (wild type) or ESX-1 mutant. (2) Lysis of the phagosomal and cellular macrophage membranes is carried out by EsxA from wild-type M. tuberculosis (2a), while the ESX-1 mutant remains trapped in the alveolar macrophages in the alveolar space (2b). (3) Infection of type II pneumocytes in the alveolar epithelium (AE) by M. tuberculosis, with resulting ESAT-6-mediated lysis, allowing passage into the interstitial tissue or (3a) translocation of infected alveolar macrophage to lung interstitial tissue. (4) Translocated bacteria are ingested by and replicate within macrophages, which produce cytokines such as fractalkine. (5) Release of bacilli by necrosis of infection-dependent macrophages. (6) Recruitment of neutrophils and naive macrophages by fractalkine and infection of new macrophages and other cells by phagocytosis. (7) Intracellular replication of bacilli in recruited infected macrophages. (8) Continuation of the cycle, leading to egress of M. tuberculosis from the host cells into deeper interstitial tissue and dissemination within the lungs. (9) Establishment of granulomas and necrosis. (Insets) (a) Electron micrograph of EsxAB. (b) Electron micrograph of the ESX-1 mutant (blue arrow) trapped within the phagosome of an alveolar macrophage in alveolar space in a murine model. (c) Electron micrograph showing wild-type M. tuberculosis (red arrow) following egress to the cytoplasm and interstitial spaces in the murine lung.