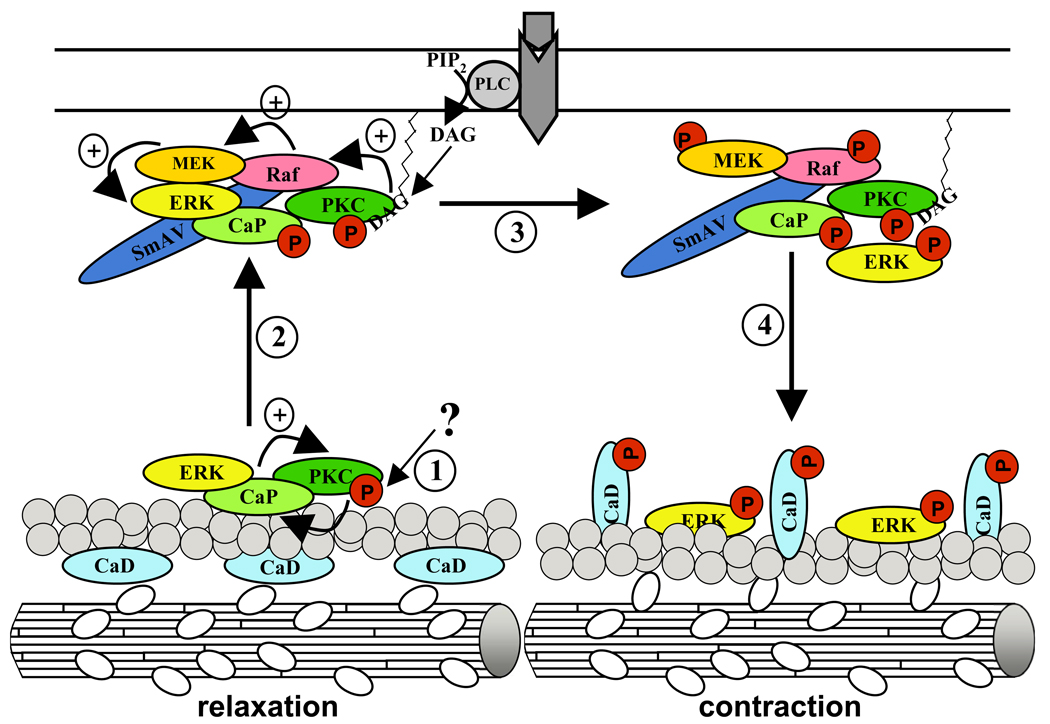
Figure 5. Model of bCaP function in regulation of smooth muscle cell contractility.
(1) Starting with a yet unidentified stimulus, PKC alpha/epsilon gets subsequently activated, an event that is further supported by bCaP binding. PKC alpha/epsilon may now phosphorylate bCaP, leading to an impaired actin binding property of bCaP. (2) Hence the ERK1/2 - PKC alpha/epsilon - bCaP complex translocates to the cell cortex where it binds to SmAV, a protein acting as a scaffold for Raf and MEK. Moreover the PKC alpha/epsilon molecule gets fully activated by membrane bound DAG that is produced by activated PLC coupled to GPCR. The activated PKC alpha/epsilon molecule phosphorylates Raf, which in turn phosphorylates MEK that now activates ERK1/2. Whereas the SmAV – bCaP – PKC alpha/epsilon complex stays at the membrane, (4) activated ERK1/2 moves back to the actin filaments where it comes in contact with its substrate h-CaD. Phosphorylation of the h-CaD molecule leads to its conformational change, resulting in enabled actin – myosin interaction and hence to contraction. For detailed information see text/article.