Figure 3.
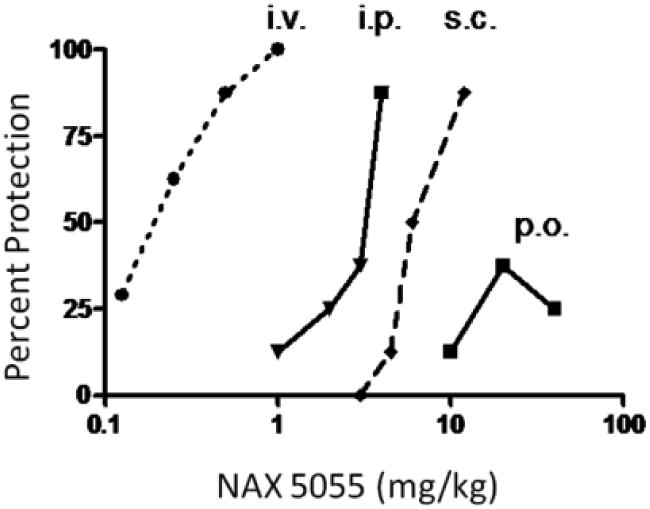
Bioavailability of NAX 5055 following several routes of systemic administration. NAX 5055 was administered intravenously (i.v.), intraperitoneally (i.p.), subcutaneously (s.c.) and orally (p.o.) in CF-1 mice. Dose-response data were generated at the following time-points for each route of administration 1 h (i.v., i.p., s.c.) and 2 hr (p.o.).
