Abstract
Objective
Developing a tissue-engineered small-diameter (<6 mm) vascular graft for reconstructive surgery has remained a challenge for the past several decades. This study was conducted to develop a decellularized umbilical artery and to evaluate its composition, endothelial cell compatibility, mechanical properties, and in vivo stability for potential use as a small-diameter vascular graft.
Methods and Results
Human umbilical arteries were isolated and decellularized by incubation in CHAPS and sodium dodecyl sulfate buffers followed by incubation in endothelial growth media-2. Decellularized umbilical arteries were completely devoid of cellular and nuclear material while retaining the integrity of extracellular collagenous matrix. The mechanical strength of the decellularized umbilical artery as assessed by its burst pressure in vitro showed no significant change from its native form. Decellularized umbilical arteries supported endothelial adherence as indicated by the re-endotheliazation with a monolayer of human umbilical vein endothelial cells. Furthermore, decellularized vessels that were implanted into nude rats as abdominal aorta interposition grafts remained mechanically intact and patent for up to 8 weeks.
Conclusion
Decellularized human umbilical arteries preserved the extracellular matrix, supported endothelialization, and retained function in vivo for up to 8 weeks. These properties suggest the potential use of decellularized umbilical arteries as small-diameter vascular grafts.
Introduction
Cardiovascular disease, including coronary artery and peripheral vascular disease, is the leading cause of mortality in the United States and accounted for over 30% deaths in 2004.1 The standard procedure to replace the diseased segments of small-diameter arteries (<6 mm) involves the use of native, autologous grafts such as saphenous veins.2,3 However, this approach requires multiple surgical procedures and is often limited by the amount of adequate autologous vessels available. Synthetic materials, including Dacron and expanded polytetrafluoroethylene (ePTFE), have been used as vascular grafts under conditions when patients do not have suitable autologous vessels, but high rate of thrombosis formation has limited the use of these synthetic grafts as small-diameter vascular grafts.4–6 In efforts to overcome these limitations, several tissue engineering approaches have been developed to prepare functional small-diameter vessels in vitro. These techniques include culturing vascular cells on a collagen matrix,7 other biodegradable scaffolds,8 or without exogenous scaffold.9 However, these tissue-engineered vascular grafts either lack sufficient mechanical strength7 or require long culture periods to obtain the mechanical strength that is required for implantation8,10,11 and are thus cost- and labor-intensive.
Recently, an alternative tissue engineering approach involving the decellularization of native tissues has shown success in a variety of applications.12,13 Decellularized tissues are composed of natural extracellular matrix and have the advantage of maintaining the structure and mechanical properties of native tissues.14–17 Decellularized biomaterials can be seeded with various cardiovascular cells, including endothelial cells, progenitor cells, and myocardial cells, to generate functional tissues, including blood vessels and other tissues.17–22 Decellularization of naturally derived tissues also reduces their immunogenicity,23–27 rendering them more favorable for allogenic use. Additionally, decellularized tissues have the potential for repair, growth, and remodeling in vivo.20,28–30 These findings suggest that the decellularization of naturally available biomaterials is a promising approach to prepare small-diameter vascular grafts.
The human umbilical cord normally contains two umbilical arteries and one umbilical vein that are embedded within a loose, proteoglycan-rich matrix known as Wharton’s jelly. The umbilical veins had been clinically used as glutaraldehyde cross-linked grafts for the last three decades with some success for lower-extremity revascularization.31–33 Umbilical veins have also been decellularized and shown potential use as vascular grafts.16 In comparison, the umbilical artery, which has an inner diameter of about 1.5 mm under collapsed conditions, has attracted little attention.34,35 Herein, we described the decellularization of human umbilical arteries and investigated the composition, mechanical properties, and endothelial cell compatibility of the decellularized vessels. The in vivo stability of the decellularized umbilical arteries was also investigated, and our results suggest that decellularization of umbilical arteries might provide a novel approach for generating small-diameter vascular grafts for vascular reconstruction surgery.
Materials and Methods
The study was approved by the Yale University Institutional Animal Care and Use Committee. All animal care complied with the Guide for the Care and Use of Laboratory Animals. Human tissue and cell population were obtained using protocols approved by the Yale University Human Investigation Committee.
Human umbilical artery preparation
Human umbilical cords (anonymized) were obtained from Yale–New Haven Children’s Hospital (New Haven, CT). The cords were stored at 4°C immediately after delivery, and the overall storage time of the umbilical cords until processing in the laboratory did not exceed 24 h. Arteries were isolated from human umbilical cords (20–30 cm in length) using sharp dissection in a sterile fashion. Briefly, a pair of Metzenbaum scissors was inserted into the Wharton’s jelly surrounding the arteries, and the tissue was dissected from the arterial vessels. Two intact arteries were subsequently separated from the umbilical vein and stored in phosphate-buffered saline (PBS, pH 7.4; Invitrogen, Carlsbad, CA) containing penicillin 100 U/mL and streptomycin 100 μg/mL (Invitrogen) at 4°C. Isolated umbilical arteries were then cut into segments of 5 cm in length and flushed a few times with PBS. Within half an hour of extraction, umbilical arteries were either used for immediate analysis or were subjected to decellularization.
Decellularization of human umbilical arteries
Decellularization of umbilical arteries was accomplished using methods that are similar to those described previously.15,36 Briefly, four to five umbilical artery segments of 5 cm in length were incubated in 250 mL CHAPS buffer (8 mM CHAPS, 1 M NaCl, and 25 mM EDTA in PBS) for 22 h, followed by brief PBS washes. Umbilical arteries were further incubated in 250 mL sodium dodecyl sulfate (SDS) buffer (1.8 mM SDS, 1 M NaCl, and 25 mM EDTA in PBS) for 22 h, followed by a 2-day wash with PBS to completely remove the detergent. In the final step, umbilical arteries were incubated at 37°C for another 2 days in endothelial growth media-2 (EGM-2) followed by washes with PBS. All decellularization steps were carried out at 37°C with high-speed agitation under sterile conditions. EGM-2 is endothelial cell basal media (Lonza, Walkersville, MD) supplemented with EGM-2 Single Quots (Lonza) and contains 12% fetal bovine serum and penicillin 100 U/mL and streptomycin 100 μg/mL. Decellularized vessels were stored in PBS containing penicillin 100 U/mL and streptomycin 100 μg/mL at 4°C for up to 2 weeks. In other studies, the freshly isolated umbilical arteries were incubated in CHAPS and SDS buffers for 14 h, respectively, before an additional 2-day incubation with EGM-2. The decellularized vessels were examined for cellularity by hematoxylin and eosin (H&E) staining and DNA quantification.
Determination of decellularization
Histological analysis
Five-mm segments of non-decellularized and decellularized umbilical arteries were fixed in 10% neutral-buffered formalin for 1 h, embedded in paraffin, cut into 5-μm sections, and stained with H&E for nuclear material, Masson’s trichrome for collagen, or elastin-van Gieson’s (EVG) stain for elastin. Paraffin-embedded sections were also stained for collagen IV to examine the basement membrane. Briefly, slides were deparaffined, rehydrated, and blocked. Sections were then incubated with rabbit polyclonal antibody to collagen IV (1:50; Abcam, Cambridge, MA) followed by FITC-conjugated goat anti-rabbit IgG (1:100; Abcam). Slides were counterstained with DAPI, and mounted. Images were obtained using a Zeiss Axiovert 200M microscope equipped with AxioCam HR with software AxioVision Release 4.5.
DNA quantification
To evaluate the decellularization quantitatively, the DNA content of umbilical arteries was determined using the PicoGreen assay. Briefly, vessel segments were lyophilized, weighed, and digested in papain buffer (papain 125 μg/mL [Sigma, Saint Louis, MO], 5 mM cysteine-HCl, and 5 mM di-sodium EDTA in PBS) at 60°C overnight as described previously.15,37 The papain sample solution was diluted with TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 7.5; Invitrogen), incubated with an equal volume of Quant-iT™ PicoGreen® dsDNA reagent (Molecular Probes, Eugene, OR), and using a fluorometer, the fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Bacteriophage λ DNA (Invitrogen) was used as a standard.
Collagen quantification
The collagen content of umbilical arteries was determined by measuring the levels of hydroxyproline as described previously.15,38 Briefly, vessel segments that were digested in papain buffer were further digested in 6 N HCl at 110°C overnight. Samples were neutralized with NaOH and incubated with chloramine T (Mallinckrodt Baker, Phillipsburg, NJ), followed by an incubation in p-dimethylaminobenzaldehyde (Mallinckrodt Baker) for 20 min at 60°C. The hydroxyproline content was measured at a wavelength of 530 nm. Collagen was calculated as 10 times the amount of hydroxyproline.39
Scanning electron microscopy
Umbilical artery segments (5 mm in length) were cut into open patches and processed for scanning electron microscopy (SEM) as described previously.40 Briefly, patches were fixed with 1% glutaraldehyde solution in 0.1 M sodium cacodylate buffer (pH 7.4; Polysciences, Warrington, PA) for 5 min. After fixation, the samples were washed for 5 min with distilled water and dehydrated with 5-min exchanges in each of the 70%, 85%, and 95% aqueous ethanol and absolute ethanol. The samples were further immersed in hexamethyldisilazane (Polysciences) for 10 min and air-dried overnight. Dried samples were mounted, sputter-coated with gold (Cressington Sputter Coater 108 auto, Watford, UK), and examined with a Phillips XL-30 environmental scanning electron microscope (FEI, Hillsboro, OR).
Human umbilical vein endothelial cell seeding
Human umbilical vein endothelial cells (HUVECs) isolated from umbilical veins using 0.1% collagenase were cultured in EGM-2 at 37°C and 5% CO2 until reaching almost confluence. Decellularized umbilical artery segments were cut into 5×3 mm open patches and put into 12-well plates with the luminal surface facing up. These patches were coated with fibronectin in PBS at 5 μg/cm2 at 37°C for 1 h, washed with PBS, and then seeded with 50 μL of HUVECs at 2×106 cells/mL. HUVECs were allowed to adhere for 1 h at 37°C and 5% CO2, after which 4 mL of EGM-2 was added to each well. Cells were then incubated for 1–5 days at 37°C and 5% CO2. At each indicated time, recellularized vessel patches were removed from 12-well plates, rinsed twice with PBS, and fixed for SEM or histological analysis. The HUVECs were at passages 2 and 3.
Mechanical analysis
The mechanical properties of umbilical arteries before and after decellularization were determined as described previously.15,41 Briefly, vessels were attached to a flow system connected to a pressure transducer. PBS was injected into the flow system at intervals of 50 mm Hg pressure until bursting of the vessel occurred. The outer diameter at each pressure was recorded using a CCD camera (Canon XL1 Digital Video Recorder) and measured using NIH Image J. The cross-sectional area of the vessel measured from histological images was assumed constant and used for determining the vessel internal radius at each pressure. Assuming an isotropic, thick-wall cylinder, the stress (σ) and strain (ε) of the umbilical artery were calculated as follows:
| (1) |
| (2) |
where P is the pressure inside the vessel, R the vessel radius, and R0 the radius at pressure zero.42 Compliance (C) between 50–150 mmHg was calculated as a percent per 100 mmHg as
| (3) |
where Dsys and Psys are the systolic vessel diameter and pressure, respectively, and Ddias and Pdias are the diastolic diameter and pressure, respectively. A slope was calculated from the four highest values of strain in the linear part of the stress–strain curve before failure, and defined as the maximal incremental modulus (Emax).8 Previous studies in our laboratory have suggested that Emax correlates with the collagen content in the vessel.8
Implantation of human umbilical arteries in nude rats
To evaluate the mechanical strength of decellularized umbilical arteries in vivo, vessels were implanted into Foxn1rnu nude rats, 3–4 months old, weighing 300 g (Harlan Sprague Dawley, Inc. [Indianapolis, IN] or Charles River Laboratories [Boston, MA]) as abdominal aorta interposition grafts. The non-decellularized umbilical arteries were used as controls. Briefly, rats were anesthetized with an intraperitoneal ketamine/xylazine/acepromazine injection and given the anti-platelet agent clopidogrel (30 mg/kg) orally 30 min before surgery, as a single dose. The anesthetized animal was opened with a midline abdominal incision, and the infrarenal abdominal aorta was exposed under standard sterile conditions. After a single dose of intravenous heparin (1000 IU/kg), the abdominal aorta was cross-clamped and divided between the renal artery and the inferior mesenteric artery. A segment of either the non-decellularized or the decellularized umbilical artery (8–10 mm in length) was inserted into the aorta end-to-end using a 10-0 monofilament nylon suture. The abdominal aorta of the nude rat is approximately 1.0 mm in inner diameter (Fig. 7C), and the umbilical artery has an inner diameter of about 1.5 mm under collapsed conditions (zero pressure) (Fig. 8A), making end-to-end anastomosis straightforward. In addition, the graft vessel wall had sufficient suture retention strength for implantation, and no difference in the surgical handling properties was found between the non-decellularized and decellularized grafts. After confirmation of graft blood flow and hemostasis post de-clamping, the wound was closed. The animals were recovered from surgery and maintained without anti-coagulation or anti-platelet treatment postoperatively.
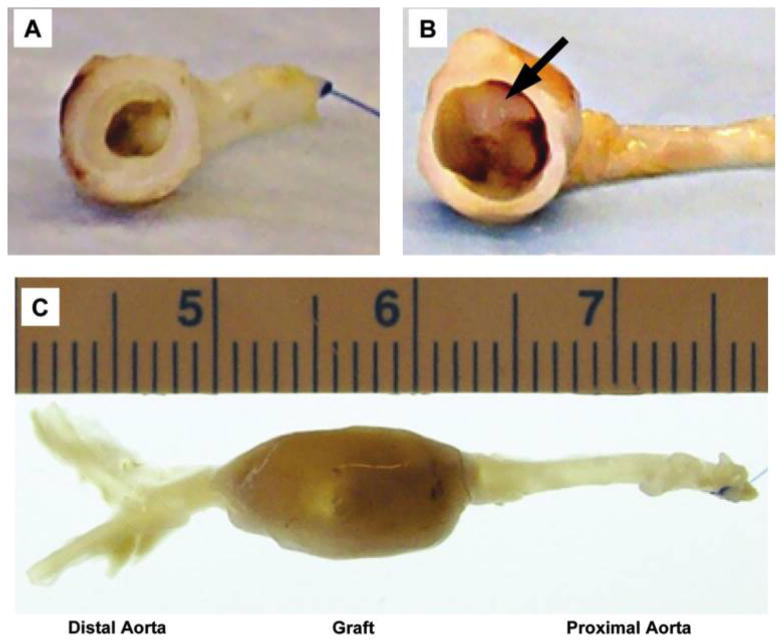
FIG. 7.
Gross-examination of the perfusion-fixed human umbilical arteries 8 weeks after implantation into nude rats as abdominal aorta interposition grafts. (A) Non-decellularized human umbilical artery; (B, C) decellularized human umbilical artery. Arrow indicates thrombosis at the proximal anastomosis. Color images available online at www.liebertonline.com/ten.
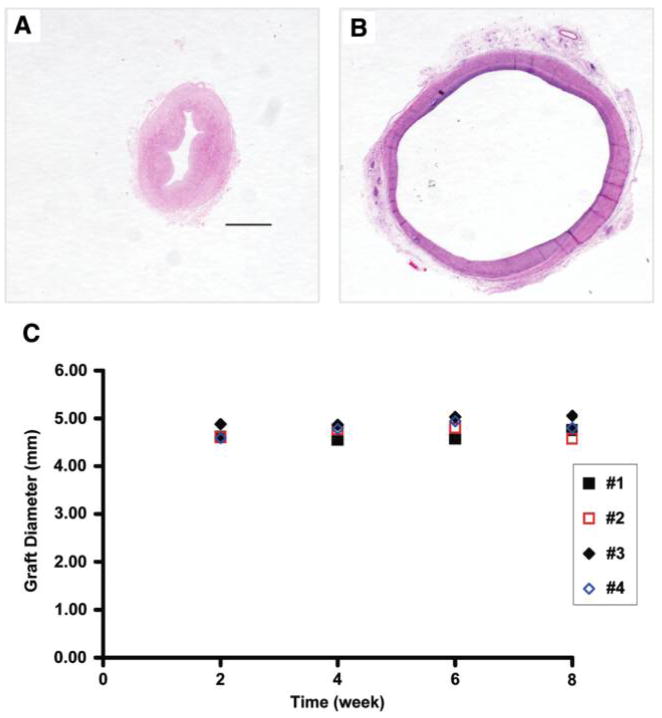
FIG. 8.
H&E staining of the decellularized umbilical arteries before implantation (A) and explanted 8 weeks after perfusion-fixation (B). Original magnification, 2.5×; scale bar =1 mm. The vessel wall was collapsed as the vessel was contracted before fixation (A). (C) Outer diameters of the decellularized umbilical arteries implanted into the nude rats (#1–#4) over an 8-week period. Color images available online at www.liebertonline.com/ten.
Assessment of graft in vivo stability and characterization of explants
At 2, 4, 6, and 8 weeks after implantation, animals were examined using a Vevo 770® Micro-ultrasound System (VisualSonics, Toronto, Canada) equipped with the RMV-704 scanhead (spatial resolution 40 μm) to determine graft patency, morphology, and blood flow. The diameter of the graft at the midpoint was measured from both transverse and longitudinal axes ultrasound images. Eight weeks postoperatively, grafts were explanted after pressure perfusion fixation at 150 mmHg with 10% formalin for 30 min. The excised grafts were fixed in 10% formalin for another 20 h.
Harvested grafts were cut into proximal, mid, and distal sections, and then embedded in paraffin, cut into 5-μm sections, and stained with H&E and Masson’s trichrome. To determine the average thickness of tissue growth within and surrounding the graft, H&E sections of the mid-graft were divided into 10 regions, and the thicknesses of tissue were measured using NIH Image J.
Paraffin-embedded sections were also analyzed by immunohistochemistry using antibodies for α-smooth muscle actin (α-SMA) to evaluate the phenotype of peri-graft tissues. Briefly, slides were deparaffined, rehydrated, and blocked. Sections were then incubated with mouse anti-human α-SMA antibody (1:1000; Dako, Carpinteria, CA) followed by biotinylated goat anti-mouse IgG (1:200; Vector Laboratories, Burlingame, CA). Bound antibodies were detected using avidin–biotin–peroxidase complex system (Vector Laboratories), and the color was visualized after incubation with NovaRed peroxidase substrate (Vector Laboratories). Slides were counterstained with hematoxylin, dehydrated, and mounted.
Statistics
Data are expressed as mean ±standard deviation (SD). Statistical significance was determined by a Student’s t-test for paired samples. Statistical significance was set at p < 0.05.
Results
Decellularized human umbilical arteries maintain extracellular collagen matrix
Freshly isolated human umbilical arteries were characterized by staining of the nuclear and cellular components (Fig. 1A), extracellular collagen matrix (Fig. 1D), and elastin fibers (Fig. 1G). After treating the freshly isolated umbilical arteries with CHAPS and SDS buffers for 22 h, respectively, the nuclear staining appeared as a diffuse smear in the vessel wall (Fig. 1B). A further incubation with EGM-2 completely removed the nuclear material as indicated by H&E staining (Fig. 1C) and confirmed by DNA quantification (Fig. 3A). Immunostaining for α-SMA and PECAM-1 (rabbit anti-PE-CAM-1, 1:250; Santa Cruz Biotechnology, Santa Cruz, CA) confirmed a complete removal of smooth muscle cells and endothelial cells from the vessel wall of the human umbilical artery treated with CHAPS and SDS buffers for 22 h each followed by EGM-2 (data not shown). In addition, the major histocompatibility complex (MHC) was also absent in these vessels as indicated by immunostaining (rabbit anti-MHC Class I, 1:50; Santa Cruz) (data not shown). Further, β-actin was almost completely absent from these vessels treated for 22 h with detergents, whereas more β-actin remained in the vessels treated with only 14 h of CHAPS and SDS buffers (data submitted for publication43). Thus, human umbilical arteries treated with CHAPS and SDS buffers for 22 h each followed by EGM-2 for 2 days will be referred to as the decellularized vessels for all the following studies.
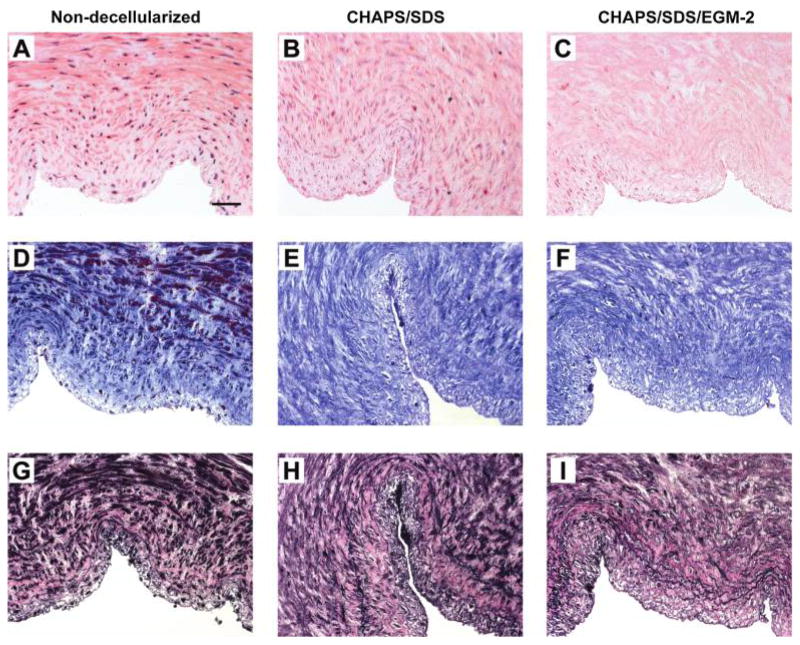
FIG. 1.
H&E staining (A–C), Masson’s trichrome staining (D–F, collagen stains blue), and EVG staining (G–I, elastin stains black) of the human umbilical arteries before (A, D, G) and after (B, E, H) incubation with CHAPS and SDS buffers for 22 h, respectively, and after further incubation with EGM-2 for 2 days (C, F, I). Original magnification, 20 ×; scale bar =50 μm. Color images available online at www.liebertonline.com/ten.
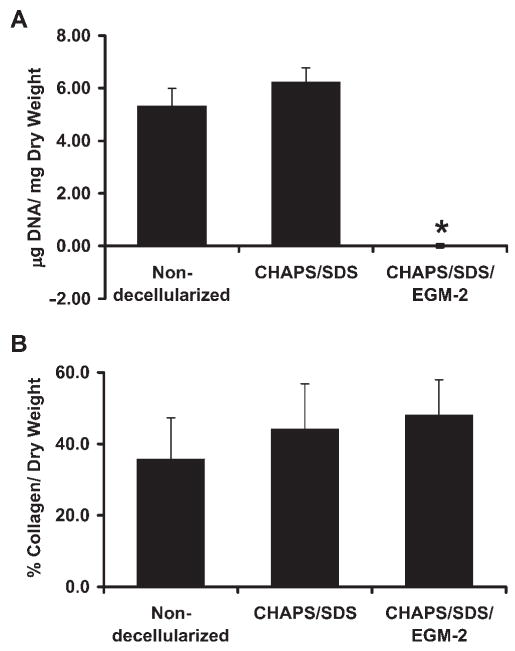
FIG. 3.
Average DNA (A) and collagen (B) per dry weight of the human umbilical arteries before decellularization, after CHAPS buffer and SDS buffer treatment, and after further incubation with EGM-2 from three independent experiments. Data are presented as mean ±SD. *Significantly different from the fresh vessels, p < 0.01.
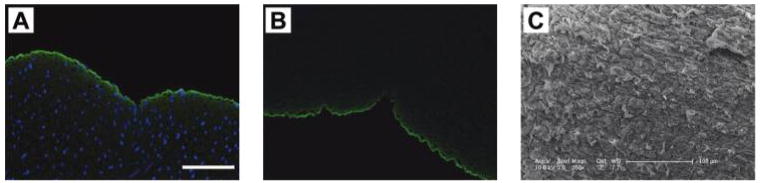
Staining of the human umbilical arteries before and after decellularization with Masson’s trichrome for collagen indicates that the extracellular collageneous matrix was well preserved in the decellularized vessels (Fig. 1F). The basement membranes of the decellularized vessels remained intact as indicated by both the staining of collagen IV, a major component of the basement membrane, and the SEM examination of the luminal surface of the vessel (Fig. 2). Quantification of collagen further indicated that the amount of collagen in the decellularized umbilical arteries was not statistically different compared to that in the non-decellularized vessels (Fig. 3B). The (nonsignificant) trend toward increasing collagen values as a percentage of dry weight after decellularization likely reflects the loss of cell mass in the tissue, and hence the relative decrease in the dry weight of the sample. However, staining with EVG indicated that some depletion of elastin might have occurred in the decellularized vessels (Fig. 1I), which might affect vessel mechanical properties at low pressures.42,44,45
FIG. 2.
Immunofluorescent staining of collagen IV (green) in the human umbilical arteries before (A) and after (B) decellularization. Some collagen autofluorescence (green) is observed in the vessel wall. Nuclei (blue) are stained with DAPI. Original magnification, 20×; scale bar =100 μm. (C) Representative SEM image of the luminal surface of the human umbilical arteries after decellularization. Scale bar =100 μm. Color images available online at www.liebertonline.com/ten.
Mechanical properties of decellularized human umbilical arteries
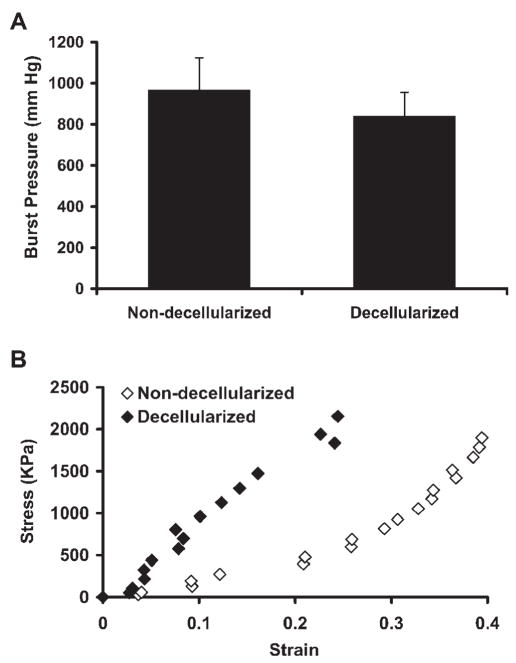
To determine whether the mechanical strength was retained in the decellularized umbilical arteries, stress–strain curves were obtained before and after decellularization as described in Materials and Methods. As shown in Fig. 4A, the non-decellularized vessels had an average maximum burst pressure at 969.66 ±154.42 mmHg, which was slightly decreased but not significantly altered in the decellularized vessels (840.37 ±114.67 mmHg, p not significant). The decellularized umbilical arteries maintained a similar maximum slope of the stress–strain curve as the non-decellularized vessels, reflecting the intact nature of the collagenous matrix, which is responsible for the stress–strain behavior at high wall stress42 (Fig. 4B). However, there was a shift in the stress–strain curve of the decellularized vessel to the left, probably due to the removal of smooth muscle cells as well as some depletion of elastin by decellularization process, which diminished the compliance of the acellular tissues at low pressures42,44,45 (Fig. 4B). No significant difference in the maximum stress, maximum modulus, and compliance were found between the non-decellularized and decellularized umbilical arteries (Table 1). These data indicate that the decellularization process did not have a significant impact on the mechanical properties of the human umbilical arteries, particularly at high wall stress.
FIG. 4.
Mechanical properties of the human umbilical arteries. (A) Average burst strength for the human umbilical arteries before and after decellularization from four independent experiments. Data are presented as mean ±SD. No significant difference was found between the non-decellularized and decellularized vessels. (B) Representative stress–strain curve of the non-decellularized and decellularized human umbilical arteries. Stress and strain values were calculated using Equations 1 and 2, respectively.
Table 1.
Mechanical Properties of Human Umbilical Arteries Before and After Decellularization from Three Independent Experiments
| Non-decellularized vessels | Decellularized vessels | p-Value | |
|---|---|---|---|
| Max. stress (kPa) | 1372.23 ±809.30 | 1618.21 ±691.26 | 0.753 |
| Max. modulus (MPa) | 13.33 ±6.85 | 7.41 ±3.85 | 0.438 |
| Compliance (% per 100 mmHg) | 5.84 ±3.10 | 4.26 ±2.96 | 0.636 |
Data are presented as mean ±SD.
Endothelial cell compatibility of the decellularized umbilical artery
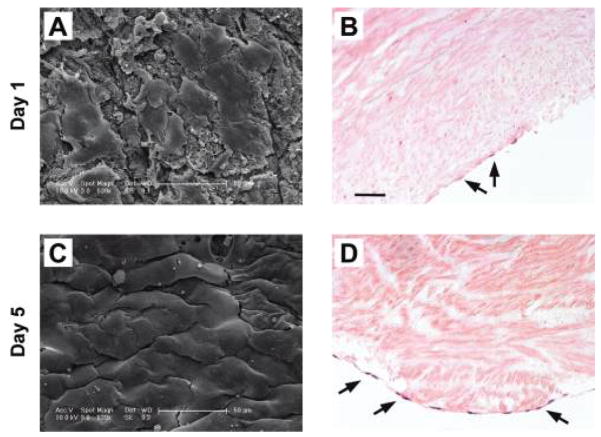
An ideal vascular graft should support endotheliazation because a confluent, functional endothelial layer has been shown to be essential for preventing graft thrombosis.46 To test whether the decellularized human umbilical arteries support endotheliazation, HUVECs were seeded onto the luminal surface of decellularized umbilical artery patches and incubated for various times. SEM examination showed that HUVECs adhered and spread well on the surface of the decellularized umbilical artery patches as early as 1 day after seeding (Fig. 4A). After a 5-day incubation period, HUVECs were fully spread and formed a nearly confluent layer (Fig. 5C, D). The HUVEC monolayer was confined to the luminal surface (Fig. 5). These data support the potential use of decellularized umbilical arteries as bio-scaffolds for vascular tissue engineering.
FIG. 5.
Seeding of the human umbilical vein endothelial cells on the decellularized umbilical arteries for 1 day (A, B) or 5 days (C, D). (A, C) representative SEM images of HU-VECs on the luminal surface of the umbilical artery. (B, D) Representative H&E staining. Arrows indicate HUVECs on the luminal surface of the recellularized human umbilical artery. Original magnification, 20×; scale bar =50 μm. Color images available online at www.liebertonline.com/ten.
In vivo durability of decellularized umbilical arteries
To evaluate in vivo stability, segments of the decellularized vessels were implanted into nude rats as abdominal interposition grafts. The freshly isolated, non-decellularized vessels served as controls. The nude rats were utilized to obviate any potential effect of the immune reaction to human xeno-grafts. Of note, the decellularized vessels were not seeded with endothelium before implantation. All rats that were implanted with the non-decellularized vessels survived after surgery (n =4). In the animals that were implanted with the decellularized vessels, however, five died within a few hours after surgery, and six survived. Development of thrombosis in those five rats implanted with the decellularized grafts was confirmed by opening up the rats immediately after death and visually checking the implanted grafts (data not shown). The incidence of acute thrombosis was presumably due to the lack of endothelium and the exposure of the collagen vessel surface to the non-anticoagulated flowing blood.
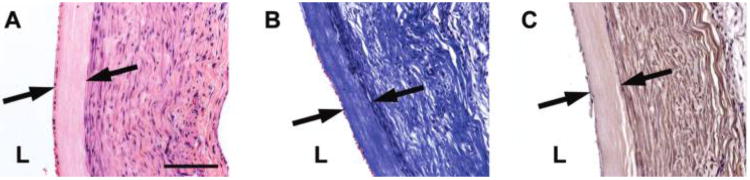
Six animals survived initial implantation with decellularized grafts, and were followed out to varying time points. To determine the short-term behavior of decellularized grafts, one rat was euthanized 2 weeks postoperatively, and the graft was explanted for histological examination. The decellularized umbilical artery graft remained intact and patent after 2 weeks of implantation (Fig. 6).
FIG. 6.
Characterization of the decellularized human umbilical artery graft 2 weeks after implantation. (A) H&E staining, (B) Masson’s trichrome staining for collagen, and (C) immunostaining for α-SMA. L, vessel lumen. Original magnification, 20×; scale bar =100 μm. Arrows mark the edges of the implanted graft. Color images available online at www.liebertonline.com/ten.
For all the remaining grafts, both the cellular controls (n =4) and decellularized grafts (n =5) remained patent throughout 8 weeks of study (Fig. 7A, B). There was no rupture or observable aneurysm formation. The decellularized umbilical artery had an inner diameter of about 1.5 mm (at zero pressure) before implantation (Fig. 8A), which matches the diameter of rat aorta (about 1 mm) (Fig. 7C). However, the graft distended in vivo immediately upon implantation to approximately 4.5 mm diameter (example of explanted vessel in Figs. 7C and 8B). Due to diameter mismatch, ultrasound examination of the aorta revealed disturbed flow near the anastomosis region and a significantly lower flow rate in the graft as compared to the native aorta.
To determine whether graft diameter remained constant in vivo during 8 weeks of arterial implantation, we undertook ultrasound examinations of all the decellularized grafts every 2 weeks. We noted from in vitro perfusion of the decellularized umbilical arteries that the vessels expand to 4.5 mm diameter at a pressure of 100 mmHg (data not shown), which we took as a starting diameter at time of implantation into the rat aorta. From the ultrasound diameter measurements (Fig. 8C), it is clear that the diameter of the decellularized grafts remained constant during the 8 weeks of arterial implantation. These data indicate that the decellularized umbilical arteries were mechanically sound for 8 weeks, and withstood physiological pressure in vivo without dilatation.
Thrombosis was noted at the proximal anastomosis in the decellularized grafts (Fig. 7B) that occurred as early as 2 weeks after implantation (data not shown). Thrombosis was not found in the mid or distal section of the decellularized grafts. In comparison, the non-decellularized control grafts did not develop any obvious thrombosis during the 8 weeks of implantation time (data not shown).
Characterization of explanted umbilical artery grafts
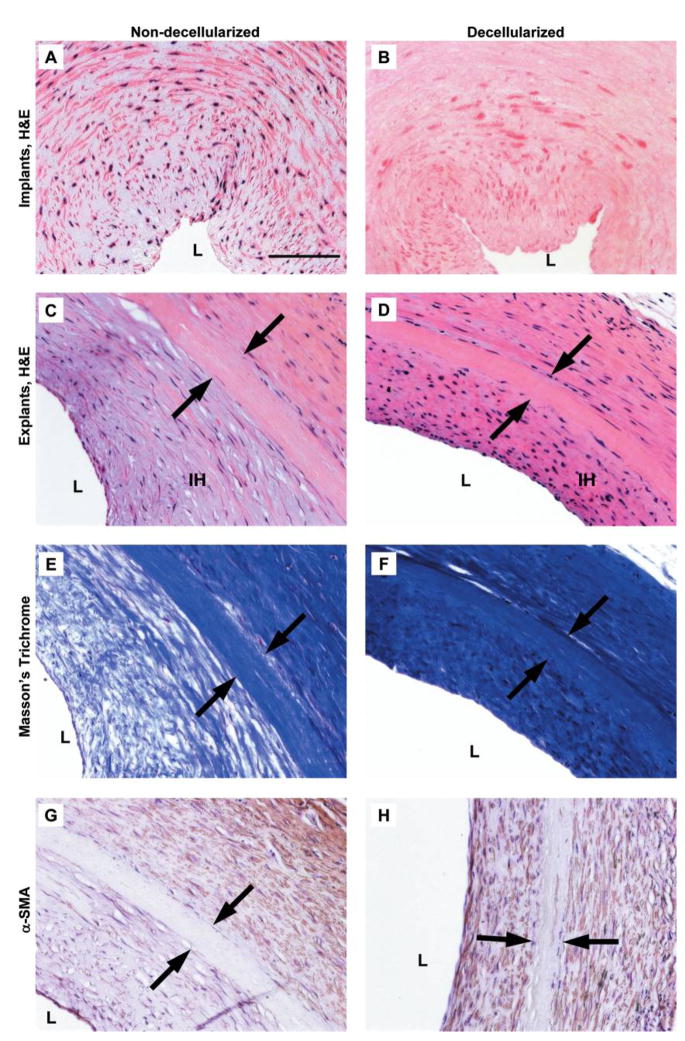
As early as 2 weeks postoperatively, cells stained positive for α-SMA, indicating a smooth-muscle phenotype, were recruited outside the surface of decellularized graft (Fig. 6). By 8 weeks postoperatively, significant amounts of collagen matrix were still present in both the decellularized and control grafts (Fig. 9E, F). Smooth muscle cells were recruited both within and outside the surface of the decellularized grafts (Fig. 9D, H). Extensive collagen matrix was found on the luminal and abluminal surfaces of the decellularized grafts, suggesting that rat smooth muscle cells might be recruited, which then proliferated and produced collagen matrix, contributing to mechanical integrity of the graft. It appeared that at 8 weeks postoperatively, most cellular recruitment was on the luminal and abluminal surfaces of the decellularized grafts, while few host cells appeared to invade the thin decellularized graft wall (Fig. 9D). Smooth muscle cells and collagen matrix were similarly deposited on the control non-decellularized grafts (Fig. 9E, G). Immunostaining for PECAM-1 indicated the presence of endothelial cells in both the non-decellularized controls and the decellularized grafts at 8 weeks postoperatively (data not shown). Interestingly, the control grafts appeared acellular 8 weeks after implantation (Fig. 9C), probably due to the apoptosis of smooth muscle cells induced by vessel wall stretching and mechanical overloading.47 (In the umbilical cord, arteries are embedded in Wharton’s jelly and experience much lower pressures than those in the rat aorta.48) The presence of smooth muscle cells and collagen matrix on the graft surface suggests the potential of graft remodeling over a long-term implantation, and implies that the source of luminal smooth muscle cells may be from the circulation, or ultimately from the host bone marrow.
FIG. 9.
Characterization of the human umbilical arteries before and after implantation. H&E staining of the non-decellularized (A, C) and decellularized vessels (B, D) before implantation (A, B) and 8 weeks postoperatively (C, D). L, vessel lumen; IH, intimal hyperplasia. Original magnification, 20×; scale bar =100 μm. Arrows mark the edges of the implanted grafts. Masson’s trichrome of the non-decellularized (E) and decellularized vessels (F) 8 weeks after implantation. Immunostaining for α-SMA of the non-decellularized (G) and decellularized vessels (H) 8 weeks postoperatively. Color images available online at www.liebertonline.com/ten.
Intimal hyperplasia, or luminal tissue ingrowth, was observed in both the controls and the decellularized grafts 8 weeks after surgery (Fig. 9). Compared to the control non-decellularized grafts, the decellularized umbilical artery grafts had a slight, but not significant (p =0.42), reduction in the average thickness of intimal hyperplasia (Fig. 10). This reduction in the intimal ingrowth was evident histologically (Fig. 9G compared to Fig. 9H).
FIG. 10.
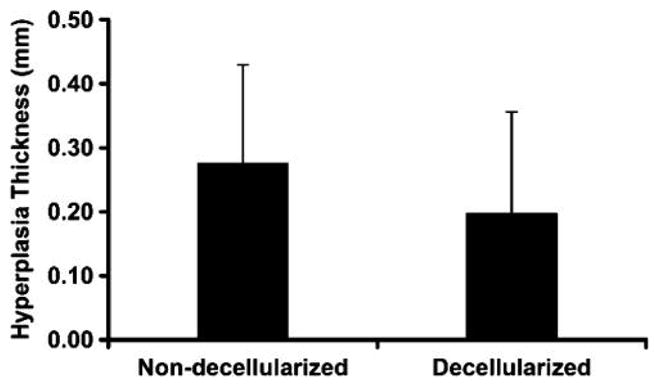
Average thickness of the intimal hyperplasia in the non-decellularized (n =4) and decellularized umbilical arteries (n =4). Data are presented as mean ±SD. No significant difference was found between the non-decellularized and decellularized grafts.
Discussion
In this study, we describe the development of a small-diameter vascular graft from human umbilical artery using a decellularization approach. An ideal tissue-engineered vascular graft should have similar mechanics to native vessels, be resistant to thrombosis, and be nonimmunogenic. Ideally, it should also be stored “on the shelf,” and perhaps seeded with autologous cells before implantation. Our results show that treating the freshly isolated, non-decellularized human umbilical artery with detergents followed by culture medium incubation quantitatively removed the cellular and nuclear materials, including MHC. The decellularized umbilical artery retained the majority of the extracellular matrix and supported the formation of a confluent endothelial layer in vitro, thus potentially allowing for endothelialization with autologous cells before implantation. The decellularized umbilical arteries also had similar mechanical properties, including maximum burst pressure and maximum modulus, to the native vessels. Furthermore, the decellularized umbilical arteries were mechanically robust in vivo for 8 weeks as an abdominal aorta interposition graft in the nude rat, with no obvious dilation or aneurysm formation. These results suggest that the decellularized umbilical artery might be of potential use as a small-diameter vascular graft for bypass surgery.
The human umbilical cord reaches a length of over 50 cm, from which two artery segments of 20–30 cm each can be easily isolated. There are no branches along this vessel. The umbilical artery has a uniform diameter throughout the whole length at about 1.5 mm under collapsed conditions and 4.5–5.5 mm under physiological pressure, once isolated from the cord. Native tissues, including human saphenous veins, have been decellularized and have shown potential use as small-diameter vascular grafts;14,17,20,28,30 however, the human umbilical artery might represent a more attractive source in that it is widely available and easily isolated. The decellularization process applied in our studies was convenient and could be easily scaled up, thus allowing the preparation of large amounts of decellularized umbilical arteries within a few days. Compared to other tissue engineering approaches, where preparation of a mechanically robust vascular graft takes weeks to months,10,11 this short production time using decellularization approaches is also cost- and labor-efficient.
Although the decellularized umbilical vein is mechanically robust in vitro,16 our experience has shown that the surgical handling properties of the decellularized vein were not as favorable as those of the decellularized artery. Human clinical studies showed that when used as an in vivo graft, the umbilical vein had to be reinforced with glutaraldehyde treatment and supported with a woven Dacron mesh to prevent vessel dilation.49 In addition, the umbilical vein has a much larger inner diameter of 3.8–5.5 mm,49 as compared to the umbilical artery (1.5 mm under zero pressure). In addition, previous studies to evaluate the in vivo behavior of the glutaraldehyde cross-linked umbilical artery in rat revealed no endothelialization on the luminal surface, while significant degeneration of the graft occurred.34 Glutaraldehyde-treated tissues have been known to be associated with altered mechanics, induction of calcification, and incomplete suppression of immunogenicity.13 Therefore, decellularization of human umbilical arteries might represent a more appropriate approach for preparing small-diameter vascular grafts.
The potential to grow, repair, and remodel might allow the graft to optimize physical properties, promote biological function, and minimize degradation over time. Decellularized grafts have been shown to induce cell infiltration and matrix remodeling in several animal models.20,28–30 In this study, we observed deposition of smooth muscle cells onto the adventitial surface of the umbilical artery graft as early as 2 weeks after implantation, and by 8 weeks postoperatively there was also organized collagen matrix deposition. Although some previous studies have shown significant cell infiltration into decellularized grafts by 8 weeks,20,28,30 we did not observe obvious cell infiltration over this time frame. These differences may be animal model dependent. In another study where a decellularized pulmonary artery tissue was implanted into a sheep, extensive cell infiltration was not observed until 20 weeks postimplantation.29 Histological evaluation of the explanted umbilical artery showed a very condensed collagen matrix, which might limit the rate of cell infiltration into the graft. It is very possible that cell infiltration might occur in the decellularized umbilical artery graft over longer time points.
Intimal hyperplasia is defined as the thickening and abnormal expansion of the intimal layer of the vessel due to cell proliferation and matrix deposition, and accounts for a significant fraction of long-term graft failures.50 Mechanisms underlying the induction of intimal hyperplasia include hemodynamic factors such as flow disruption and injury of the endothelial layer.50,51 Interestingly, the decellularized graft had a trend of decreased intimal hyperplasia formation as compared to the non-decellularized control. Intimal hyperplasia is thought to be induced by the migration of medial smooth muscle cells and adventitial fibroblasts into the intimal region,50,52,53 suggesting that decellularization of the umbilical artery might limit the cell sources for intimal hyperplasia formation. Obviously, future studies using a different animal model with appropriate hemodynamic conditions are required to evaluate whether intimal hyperplasia is significantly reduced in the decellularized umbilical arteries as compared to cellular grafts.
The non-decellularized control human umbilical arteries when implanted into nude rats as abdominal grafts had no occlusion or thrombosis. In comparison, some of the decellularized umbilical artery grafts were occluded within 24 h after implantation, leading to higher mortality rate in those animals. The decellularized umbilical artery grafts also developed various levels of thrombosis at the proximal anastomosis sites during an 8-week implantation period. A confluent endothelial layer plays an important role in improving graft patency, by secreting nitric oxide, prostacyclins, and tissue-plasminogen activator, for example.8,21,46 Hence, the tendency toward clotting and thrombosis in the decellularized vessels as compared to the non-decellularized umbilical arteries is likely due to the lack of an endothelium. Because our data have shown that the decellularized umbilical arteries supported HUVEC seeding in vitro, future studies will be performed to determine whether pre-seeding the luminal surface of the decellularized umbilical artery with an endothelial layer will prevent the early occlusion and decrease the incidence of thrombosis.
Various approaches, including physical, chemical, and enzymatic treatment, have been developed for tissue decellularization.12 Although previous studies have demonstrated significant DNA removal from native porcine vessels after the treatment with CHAPS and SDS buffers,15 our data indicate that the nuclear material was only completely removed after an additional EGM-2 incubation. In our studies to further evaluate the role of EGM-2 in decellularization, we found that EGM-2 incubation had no effect on removing cellular proteins from CHAPS buffer–treated and SDS buffer–treated tissues,43 suggesting that some nuclease activities in EGM-2 might contribute to its DNA removal. Among the potential biochemical mediators for DNA removal, fetal bovine serum, one of the constituents of EGM-2 contains nucleases that may facilitate removal of DNA from the tissues.43 Compared to other decellularization methods that utilize harsh enzymatic reagents,15 the EGM-2 incubation approach is convenient and does not impact tissue mechanical characteristics.
To summarize, decellularization of the widely available human umbilical artery provides a convenient and efficient approach for developing a potential small-diameter vascular graft. Decellularization minimizes the immunogenicity of the resultant grafts, and allows for long-term storage. Naturally derived extracellular matrix maintains many of the mechanical properties of the original tissue, and supports an endothelial cell lining. A further assessment of the decellularized umbilical artery incorporating lining the luminal surface with endothelial cells, using a larger animal model for an appropriate anastomotic match, and following over a longer-term implantation, is indicated to demonstrate the potential use of the decellularized umbilical artery as a small-diameter vascular graft in humans.
Acknowledgments
The authors thank Louise Benson for providing the umbilical cords. The authors also thank Liping Zhao for technical assistance. This work was supported by NIH R01 HL083895 (L.E.N.).
Footnotes
Disclosure Statement
The authors (L.G., A.M., S.A.C., C.K.B., and L.E.N.) have no conflicts of interest to disclose for this manuscript.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Tu JV, Pashos CL, Naylor CD, Chen E, Normand SL, Newhouse JP, McNeil BJ. Use of cardiac procedures and outcomes in elderly patients with myocardial infarction in the United States and Canada. N Engl J Med. 1997;336:1500. doi: 10.1056/NEJM199705223362106. [DOI] [PubMed] [Google Scholar]
- 3.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, Hart JC, Herrmann HC, Hillis LD, Hutter AM, Jr, Lytle BW, Marlow RA, Nugent WC, Orszulak TA. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110:e340. [PubMed] [Google Scholar]
- 4.Teebken OE, Haverich A. Tissue engineering of small diameter vascular grafts. Eur J Vasc Endovasc Surg. 2002;23:475. doi: 10.1053/ejvs.2002.1654. [DOI] [PubMed] [Google Scholar]
- 5.Stephen M, Loewenthal J, Little JM, May J, Sheil AG. Autogenous veins and velour dacron in femor-opopliteal arterial bypass. Surgery. 1977;81:314. [PubMed] [Google Scholar]
- 6.O’Donnell TF, Jr, Mackey W, McCullough JL, Jr, Maxwell SL, Jr, Farber SP, Deterling RA, Callow AD. Correlation of operative findings with angiographic and noninvasive hemodynamic factors associated with failure of polytetrafluoroethylene grafts. J Vasc Surg. 1984;1:136. doi: 10.1067/mva.1984.avs0010136. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 8.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 9.L’Heureux N, Germain L, Labbe R, Auger FA. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vasc Surg. 1993;17:499. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 10.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, Conroy N, Jones R, Vasanawala A, Sanzgiri S, Langer R. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 11.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 14.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, Dimuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659. [PubMed] [Google Scholar]
- 16.Daniel J, Abe K, McFetridge PS. Development of the human umbilical vein scaffold for cardiovascular tissue engineering applications. ASAIO J. 2005;51:252. doi: 10.1097/01.mat.0000160872.41871.7e. [DOI] [PubMed] [Google Scholar]
- 17.Amiel GE, Komura M, Shapira O, Yoo JJ, Yazdani S, Berry J, Kaushal S, Bischoff J, Atala A, Soker S. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 2006;12:2355. doi: 10.1089/ten.2006.12.2355. [DOI] [PubMed] [Google Scholar]
- 18.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, Park HY, Jang Y, Chang BC, Choi CY, Hwang KC, Kim BS. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 20.Borschel GH, Huang YC, Calve S, Arruda EM, Lynch JB, Dow DE, Kuzon WM, Dennis RG, Brown DL. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005;11:778. doi: 10.1089/ten.2005.11.778. [DOI] [PubMed] [Google Scholar]
- 21.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teebken OE, Bader A, Steinhoff G, Haverich A. Tissue engineering of vascular grafts: human cell seeding of decellularised porcine matrix. Eur J Vasc Endovasc Surg. 2000;19:381. doi: 10.1053/ejvs.1999.1004. [DOI] [PubMed] [Google Scholar]
- 23.Allaire E, Bruneval P, Mandet C, Becquemin JP, Michel JB. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery. 1997;122:73. doi: 10.1016/s0039-6060(97)90267-1. [DOI] [PubMed] [Google Scholar]
- 24.Meyer SR, Nagendran J, Desai LS, Rayat GR, Churchill TA, Anderson CC, Rajotte RV, Lakey JR, Ross DB. Decellularization reduces the immune response to aortic valve allografts in the rat. J Thorac Cardiovasc Surg. 2005;130:469. doi: 10.1016/j.jtcvs.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Rieder E, Nigisch A, Dekan B, Kasimir MT, Muhlbacher F, Wolner E, Simon P, Weigel G. Granulocyte-based immune response against decellularized or glutaraldehyde cross-linked vascular tissue. Biomaterials. 2006;27:5634. doi: 10.1016/j.biomaterials.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Wells W, Malas M, Baker CJ, Quardt SM, Barr ML. Depopulated vena caval homograft: a new venous conduit. J Thorac Cardiovasc Surg. 2003;126:498. doi: 10.1016/s0022-5223(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 27.Elkins RC, Lane MM, Capps SB, McCue C, Dawson PE. Humoral immune response to allograft valve tissue pretreated with an antigen reduction process. Semin Thorac Cardiovasc Surg. 2001;13:82. [PubMed] [Google Scholar]
- 28.Huynh T, Abraham G, Murray J, Brockbank K, Hagen PO, Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol. 1999;17:1083. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- 29.Ketchedjian A, Jones AL, Krueger P, Robinson E, Crouch K, Wolfinbarger L, Jr, Hopkins R. Recellularization of decellularized allograft scaffolds in ovine great vessel reconstructions. Ann Thorac Surg. 2005;79:888. doi: 10.1016/j.athoracsur.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 30.Martin ND, Schaner PJ, Tulenko TN, Shapiro IM, Dimatteo CA, Williams TK, Hager ES, DiMuzio PJ. In vivo behavior of decellularized vein allograft. J Surg Res. 2005;129:17. doi: 10.1016/j.jss.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Dardik H, Miller N, Dardik A, Ibrahim I, Sussman B, Berry SM, Wolodiger F, Kahn M, Dardik I. A decade of experience with the glutaraldehyde-tanned human umbilical cord vein graft for revascularization of the lower limb. J Vasc Surg. 1988;7:336. doi: 10.1067/mva.1988.avs0070336. [DOI] [PubMed] [Google Scholar]
- 32.Dardik H. The second decade of experience with the umbilical vein graft for lower-limb revascularization. Cardiovasc Surg. 1995;3:265. doi: 10.1016/0967-2109(95)93874-o. [DOI] [PubMed] [Google Scholar]
- 33.Dardik H, Wengerter K, Qin F, Pangilinan A, Silvestri F, Wolodiger F, Kahn M, Sussman B, Ibrahim IM. Comparative decades of experience with glutaraldehyde-tanned human umbilical cord vein graft for lower limb revascularization: an analysis of 1275 cases. J Vasc Surg. 2002;35:64. [PubMed] [Google Scholar]
- 34.Yeh HS, Keller JT, Brackett KA, Frank E, Tew JM., Jr Human umbilical artery for microvascular grafting. Experimental study in the rat. J Neurosurg. 1984;61:737. doi: 10.3171/jns.1984.61.4.0737. [DOI] [PubMed] [Google Scholar]
- 35.Kerdjoudj H, Boura C, Marchal L, Dumas D, Schaff P, Voegel JC, Stoltz JF, Menu P. Decellularized umbilical artery treated with thin polyelectrolyte multilayer films: potential use in vascular engineering. Biomed Mater Eng. 2006;16:S123. [PubMed] [Google Scholar]
- 36.Livesey SA, Del Campo AA, Nag A, Nichols KB, Coleman C. LifeCell Corporation. The Woodlands; TX: 1994. Method for processing and preserving collagen-based tissues for transplantation. US Patent 5336616. [Google Scholar]
- 37.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 38.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 39.Piez K, Likins RC. The nature of collagen. In: Sognnaes RF, editor. Calcification in biological systems; A symposium presented at the Washington meeting of the American Association for the Advancement of Science; December 29, 1958; American Association for the Advancement of Science; 1960. pp. 411–420. [Google Scholar]
- 40.Nation JL. A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol. 1983;58:347. doi: 10.3109/10520298309066811. [DOI] [PubMed] [Google Scholar]
- 41.Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armentano RL, Levenson J, Barra JG, Fischer EI, Breitbart GJ, Pichel RH, Simon A. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am J Physiol. 1991;260:H1870. doi: 10.1152/ajpheart.1991.260.6.H1870. [DOI] [PubMed] [Google Scholar]
- 43.Gui L, Chan SA, Breuer CK, Niklason LE. Novel utilization of serum in tissue decellularization. Tissue Engineering. doi: 10.1089/ten.tec.2009.0120. (submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bank AJ, Wang H, Holte JE, Mullen K, Shammas R, Kubo SH. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation. 1996;94:3263. doi: 10.1161/01.cir.94.12.3263. [DOI] [PubMed] [Google Scholar]
- 45.Barra JG, Armentano RL, Levenson J, Fischer EI, Pichel RH, Simon A. Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res. 1993;73:1040. doi: 10.1161/01.res.73.6.1040. [DOI] [PubMed] [Google Scholar]
- 46.Rashid ST, Salacinski HJ, Fuller BJ, Hamilton G, Seifalian AM. Engineering of bypass conduits to improve patency. Cell Prolif. 2004;37:351. doi: 10.1111/j.1365-2184.2004.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Best PJ, Hasdai D, Sangiorgi G, Schwartz RS, Holmes DR, Jr, Simari RD, Lerman A. Apoptosis. Basic concepts implications and in coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:14. doi: 10.1161/01.atv.19.1.14. [DOI] [PubMed] [Google Scholar]
- 48.Woodbury RA, Robinow M, Hamilton WF. Blood pressure studies on infants. Am J Physiol. 1938;122:472. [Google Scholar]
- 49.Dardik H, Dardik II. Successful arterial substitution with modified human umbilical vein. Ann Surg. 1976;183:252. doi: 10.1097/00000658-197603000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemson MS, Tordoir JH, Daemen MJ, Kitslaar PJ. Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg. 2000;19:336. doi: 10.1053/ejvs.1999.1040. [DOI] [PubMed] [Google Scholar]
- 51.Allaire E, Clowes AW. Endothelial cell injury in cardiovascular surgery: the intimal hyperplastic response. Ann Thorac Surg. 1997;63:582. doi: 10.1016/s0003-4975(96)01045-4. [DOI] [PubMed] [Google Scholar]
- 52.Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;6:369. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- 53.Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation. 1996;93:340. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]