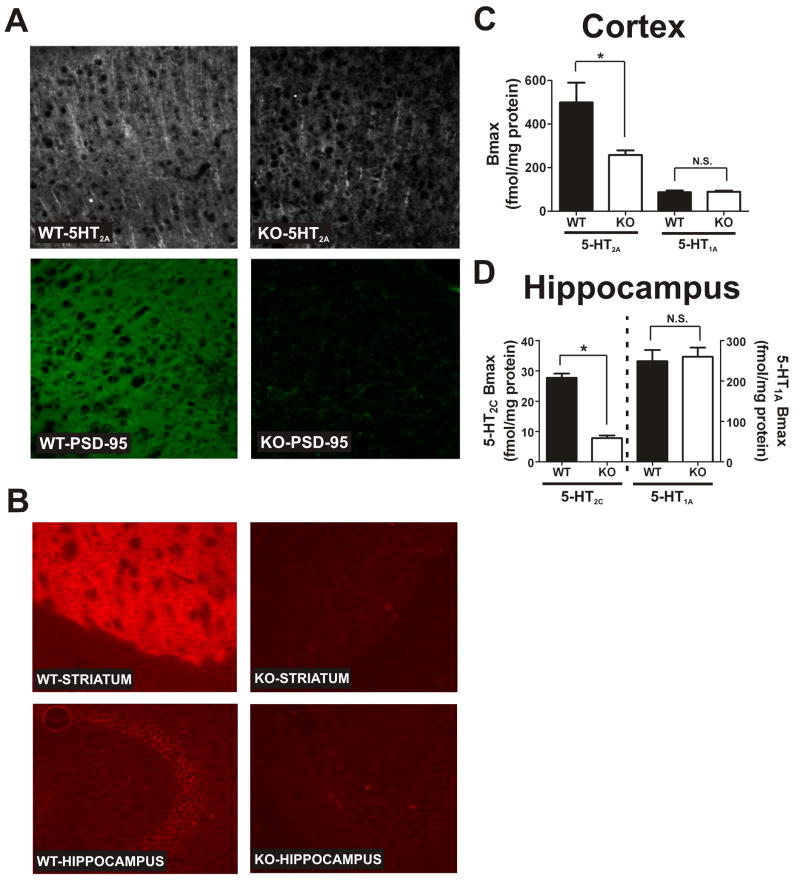
Figure 1. Genetic deletion of PSD-95 results in a selective loss of 5-HT2A and 5-HT2C receptors.
(A) 5-HT2A and PSD-95 double-label immunochemistry in medial prefrontal cortex of PSD-95wildtype and PSD-95null mice shows a large reduction in 5-HT2A receptor expression in null mice (N=3 littermate pairs). (B) 5-HT2C immunochemistry in PSD-95wildtype and PSD-95null striatum and hippocampus reveals that 5-HT2C receptor expression is almost completely abolished in the absence of PSD-95 in both striatum and hippocampus (N=3 littermate pairs). (C) Comparison of Bmax estimates for the 5-HT2A receptor (N=4 littermate pairs) and the 5-HT1A receptor (N=5 littermate pairs) in PSD-95wildtype and PSD-95null cortices. Bmax estimates were obtained by performing [3H]-ketanserin (5-HT2A) and [3H]-WAY100635 (5- HT1A) saturation binding on microdissected and homogenized cortical tissue. Quantitation showed a roughly 40% reduction in 5-HT2A expression and no change in 5-HT1A expression in the cortices of PSD-95null mice. (D) Comparison of Bmax estimates for the 5-HT2C receptor (N=3; tissue from 3 animals was pooled for each measurement, for a total of 9 animals, all littermate pairs) and 5-HT1A receptor (N=6 littermate pairs) in PSD-95wildtype and PSD-95null hippocampi. Bmax estimates were obtained by performing [3H]-mesulergine saturation binding in the presence of 100 nM spiperone to block the vast majority of 5-HT2A receptors (5- HT2C) and [3H]-WAY100635 saturation binding (5-HT1A). Quantitation showed an almost 70% reduction in 5-HT2C expression and no change in 5-HT1A expression in hippocampus in the absence of PSD-95. All saturation binding was analyzed using non-linear least squares fitting. Bmax data are presented as means +/- SEM. *p < .05, **p < .01, ***p < .001; one-tailed unpaired t-test.