Abstract
At sites of inflammation, infection or vascular injury local proinflammatory or pathogen-derived stimuli render the luminal vascular endothelial surface attractive for leukocytes. This innate immunity response consists of a well defined and regulated multistep cascade involving consecutive steps of adhesive interactions between the leukocytes and the endothelium. During the initial contact with the activated endothelium leukocytes roll along the endothelium via a loose bond which is mediated by selectins. Subsequently leukocytes are activated by chemokines presented on the luminal endothelial surface, which results in the activation of leukocyte integrins and the firm leukocyte arrest on the endothelium. After their firm adhesion, leukocytes make use of two transmigration processes to pass the endothelial barrier, the transcellular route through the endothelial cell body or the paracellular route through the endothelial junctions. In addition, further circulating cells, such as platelets arrive early at sites of inflammation contributing to both coagulation and to the immune response in parts by facilitating leukocyte-endothelial interactions. Platelets have thereby been implicated in several inflammatory pathologies. This review summarizes the major mechanisms and molecules involved in leukocyte-endothelial and leukocyte-platelet interactions in inflammation.
Keywords: Endothelial cells, Adhesion, Leukocytes, Platelets, Inflammation
Introduction
The characteristic steps taken by leukocytes to extravasate from blood to the site of inflammation caused by either exogenous or endogenous stimuli have been recognized and summarized for about two decades as the ‘three-step’ paradigm of inflammatory cell recruitment that involved rolling, activation and adhesion. Extensive research in this field has resulted in the expansion of the three-step leukocyte adhesion cascade to include further adhesive processes between leukocytes and the endothelium, such as the slow rolling, the locomotion or crawling as well as the transendothelial migration [1-10]. The interaction between the leukocytes and the endothelium comprises a variety of adhesive and migratory molecular events including low affinity transient and reversible rolling adhesions, integrin-dependent firm adhesive interactions and migratory events of the leukocytes through the endothelium and beyond that, such as the penetration of the basement membrane, and migration in the interstitial space [5, 11].
Adhesion molecules
Leukocyte-endothelial adhesion molecules can be grouped into three families. (i) Selectins are a family of three carbohydrate-recognizing molecules, of which, E-selectin is expressed on the activated endothelium, P-selectin is expressed on platelets and the endothelium, and L-selectin is constitutively expressed on leukocytes [12]. Several studies engaging antibody blockade of selectins demonstrated the participation of selectins in leukocyte rolling [13, 14]. Selectin ligands are glycoproteins that are rich in glycosylation of O-linked and N-linked carbohydrates [15, 16]. (ii) Integrins are heterodimers comprising of an alpha and a beta chain and can recognize multiple ligands including proteins of the extracellular matrix, cell surface glycoproteins as well as complement factors and soluble components of the haemostatic and fibrinolytic cascade [17-19]. Leukocytes express integrins of the β2–family (CD11/CD18). In addition, several leukocyte subpopulations express β1, β7 and α4 integrins on their surface. Integrins require conformational changes to gain full adhesive function [17, 20]. (iii) The major integrin ligands involved in leukocyte adhesion belong to the immunoglobulin superfamily [21] and include intercellular cell adhesion molecules (ICAM) 1-5, vascular cell adhesion molecule-1 (VCAM-1), as well as the junctional adhesion molecules (JAMs) [4, 5], that are expressed on endothelial and other cells. Further important adhesion receptors of the immunoglobulin superfamily involved in leukocyte recruitment are the platelet-endothelial cell adhesion molecule-1 (PECAM-1) [22] and endothelial cell adhesion molecule (ESAM) [23].
Leukocyte margination, capture and cell rolling
The very initial contact of the leukocyte with the vascular wall is determined by simple flow dynamics. Leukocyte margination is defined by the flow of leukocytes in a position close to the endothelial surface rather than in the central blood stream, depends on the interaction between individual red and white blood cells, and is enhanced in small postcapillary venules, which represent the main location for leukocyte recruitment [24]. Margination is rather a passive phenomenon and it is not entirely clear whether it is a rate-limiting step in inflammatory cell recruitment.
The initial process of active leukocyte recruitment is the tethering or rolling of leukocytes describing the initial selectin-mediated interaction between leukocytes and endothelial cells [25]. Antibody blockade of selectins inhibited leukocyte rolling in vivo in multiple studies [13, 26]. An apparent synergism between L-selectin and the vascular endothelial selectins exists [27]. Selectins bind to carbohydrate ligands, and leukocyte rolling can be inhibited by charged carbohydrates [28]. Moreover, P-selectin-/- mice show no leukocyte rolling in vivo [29]. The most important ligand for selectins is the glycoprotein P-Selectin Glycoprotein Ligand- 1 (PSGL-l), which is present as a homodimer on leukocytes and can bind to both P-selectin and E-selectin [30, 31]. Interestingly, PSGL-1 ligation in neutrophils by both P-selectin and E-selectin can result in activation of integrins, thus providing a link between rolling and the subsequent integrin-mediated firm adhesion [31, 32]. This interplay between the PSGL-1-selectin interaction and LFA-1 activation is important for slow rolling of neutrophils. It has been shown that an ITAM-dependent pathway involving the Src-family kinase Fgr and the ITAM-containing adaptor proteins DAP12 and FcRgamma mediates the signaling events downstream of PSGL-1 that are required to initiate neutrophil slow rolling [32]. The importance of the rolling process as a required step in the leukocyte recruitment cascade is underlined by a congenital pathology called leukocyte adhesion deficiency II (LAD II). Patients suffering from this rare disease reveal a congenital defect in fucose processing and cannot produce functional fucosylated selectin ligands [33]. Neutrophils from patients with this syndrome are defective in rolling [33], associated with severe recurrent bacterial infections.
Activation and adhesion of leukocytes
During the process of leukocyte rolling the contact of leukocytes with the luminal endothelial surface allows leukocytes to effectively “sense” the endothelial surface-bound chemokines. Chemokines are dramatically induced by inflammatory mediators and secreted [34]. Chemokines secreted from an inflamed environment can be transcytosed through the endothelium and presented on the endothelial cell surface associated with proteoglycans [35, 36]. These surface-deposited chemokines are presented to rolling leukocytes. By interaction with the G-protein coupled chemokine receptors on leukocytes chemokines induce intracellular signals leading to inside-out integrin activation and firm leukocyte adhesion as well as to shape change and pseudopod formation [35]. These shape changes are associated with the conversion of G-actin to F-actin enabling the cell to enter the adhesion and later the transmigration process.
Chemokines are grouped into four major subfamilies depending on the presence or absence of intervening amino acids between the first two N-terminal cystein residues and are thereby designated as CC, CXC, CX3C or C chemokines. Besides secreted chemokines, CX3CL1 (fractalkine) and CXCL16 represent the transmembrane chemokines. CXCL8 (Interleukin-8, IL-8) plays an important role for neutrophil activation as IL-8 receptor-/- mice have twelve-fold elevated systemic neutrophil counts and are severely impaired in their ability to recruit neutrophils into thioglycollate-induced peritonitis [37].
Binding of chemokines to their receptors on leukocytes results in the inside-out signaling activation of leukocyte beta1-integrins and the beta2-integrins LFA-1 (αLβ2) and Mac-l (αMβ2), that mediate firm arrest of leukocytes [38]. In humans, leukocyte adhesion deficiency I (LAD-I) is caused by the absence of beta2 integrins resulting in impaired leukocyte recruitment and as a consequence recurrent, life-threatening bacterial infections [39, 40]. In mice, beta2-integrin deficieny results in severely reduced firm leukocyte adhesion and impaired leukocyte recruitment [41]. Neutrophils use both LFA-1 (CD11a/CD18;αLβ2) and Mac-l (CD11b/CD18;αMβ2) for adhesion as shown in different animal models [18, 42, 43]. LFA-1 and Mac-1 bind to endothelial ICAMs such as ICAM-1 and ICAM-2 [44]. ICAM-1 and ICAM-2 are constitutively expressed, and ICAM-1 expression is further increased after endothelial activation [45, 46]. In contrast, endothelial VCAM-1 is recognized by beta-1 integrin receptors predominantly found on lymphocytes and monocytes [47]. This pathway of adhesion appears to be responsible for immune functions that occur in the absence of beta2 integrins in LAD-I patients [48]. The adhesive activity of integrins is regulated by alterations in integrin affinity and integrin valency, the former being mediated by conformational changes of the integrin subunits and the latter involving changes of integrin distribution on the cell surface [9, 20, 49-51].
Transmigration
Transmigration of leukocytes through the vascular endothelium can take place in a paracellular or transcellular manner [6, 10, 52]. The major determinant in the paracellular pathway is the endothelial intercellular junctions, as changes in the integrity of the endothelial barrier in postcapillary venules affect inflammatory cell recruitment [4, 53, 54]. Two types of interendothelial junctions are relevant for the transmigration process [55]. Tight junctions (zonula occludens) are apically located and contain three types of transmembrane proteins, occludin, claudins and junctional adhesion molecules (JAMs). These transmembrane molecules are linked to the actin cytoskeleton via interaction with molecules containing PDZ domains, such as ZO-1 [4]. Hierarchically the most important determinant of the endothelial barrier are the adherens junctions that are formed by the homophilic interaction of VE-Cadherin [56]. VE-cadherin acts as a gatekeeper for the passage of leukocytes and inhibition of VE-cadherin increases the permeability of endothelial-cell monolayers and the rate of neutrophil extravasation in vivo [57]. In vitro studies indicate that VE-cadherin gaps may form transiently during leukocyte diapedesis [58]. The function of VE-cadherin to regulate the endothelial barrier or leukocyte transmigration can be modulated by phosphorylation of the cytoplasmic tail of VE-cadherin, at tyrosines 658 and 731 or at Ser 665 [59, 60], which can be stimulated by ICAM-1-mediated neutrophil adhesion to endothelial cells [61].
In addition, leukocyte transmigration involves homophilic and heterophilic interactions between adhesion receptors on the the leukocyte and the endothelium [4, 10, 22, 62]. Junctional adhesion molecules (JAMs) belong to the immunoglobulin superfamily consisting of two extracellular Ig-like domains [4]. Besides interacting in a homophilic manner [63, 64], JAMs are engaged as counter-receptors for leukocyte integrins. JAM-A has been shown to interact with LFA-1 [65], JAM-B binds to VLA-4 [66] and JAM-C interacts with Mac-1 [67, 68]. The function of JAM-A in leukocyte diapedesis in vivo has been demonstrated by antibody inhibition experiments [69] as well as by evaluation of JAM-A-deficient mice showing that JAM-A on neutrophils as well as on endothelial cells participates in neutrophil extravasation [70], [71]. JAM-C can function as a heterophilic binding partner of integrin Mac-1. The JAM-C/Mac-1 interaction was found to mediate a firm platelet-neutrophil interaction [68]. In addition, soluble JAM-C or antibodies to JAM-C blocked neutrophil transmigration through endothelial cells, whereas accumulation of neutrophils in vivo was enhanced by endothelial-specific overexpression of JAM-C in mice [72, 73]. Thus, JAMs are important receptors determining leukocyte migration across the endothelial barrier [4, 74]. In addition, JAM-C also acts to antagonize VE-cadherin-dependent interendothelial adhesion thereby promoting the disruption of the endothelial barrier by a mechanism involving the small GTPase Rap1 [75].
Another important adhesion molecule regulating leukocyte transmigration is PECAM-1, a member of the immunoglobulin superfamily consisting of six Ig domains that is expressed at the intercellular borders of endothelial cells as well on platelets, neutrophils, monocytes and some T cells [22]. Several studies suggest that endothelial transmigration of leukocytes is mediated by the homophilic interaction of platelet endothelial cell adhesion molecule 1 (PECAM- I, CD31) as shown by antibody blocking studies both in vitro and in vivo [76, 77], [22, 77, 78]. Endothelial PECAM-1, which was found to act preferentially in interleukin (IL)-1beta- but not TNF-alpha-induced inflammatory cell recruitment [79], recycles between the junctions and the subjunctional plasmalemma, and is targeted to the zone of active leukocyte transmigration [78]. Recently, CD177, a 58- to 64-kDa glycosyl-phosphatidyl-inositol (GPI)-anchored glycoprotein expressed exclusively on neutrophils, was identified as a novel heterophilic adhesion ligand of PECAM-1 involved in neutrophil transendothelial migration [80]. Distal to the step of transmigration mediated by PECAM-1, a further molecule, CD99, which is expressed on both neutrophils and other leukocytes and at the interendothelial junctions, participates in leukocyte transmigration [81], [82].
Endothelial cell ICAM-1 has been implicated in transmigration. ICAM-1 can colocalize with ringlike LFA-1 clusters on leukocytes during transmigration. In addition, a “cuplike” transmigratory structure containing ICAM-1-enriched microvilli-like projections was shown to surround transmigrating neutrophils during diapedesis [83, 84]. During neutrophil adhesion to endothelial cells, ICAM-1 ligation induces cytoskeletal remodeling associated with ICAM-1 clustering, a process that is dependent on cortactin [85]. Moreover, cortactin and its tyrosine phosphorylation are required for the clustering of ICAM-1 around transmigrating neutrophils [86].
Endogenous inhibitors of leukocyte adhesion
In contrast to the numerous adhesion receptors that have been identified to promote leukocyte-endothelial interactions, very little is known about functionally important endogenous inhibitors of leukocyte adhesion [5, 8, 10, 74]. Endogenous inhibitors exist in several aspects of inflammation and immunity, and function to attenuate exuberant inflammatory and immune activation [87, 88]. Recently, developmental endothelial locus-1 (Del-1) was identified as an endogenous inhibitor of the leukocyte adhesion cascade. Del-1, a glycoprotein secreted by endothelial cells and associated with proteoglycans of the endothelial cell surface and / or with the extracellular matrix [89], has been previously implicated as an adhesive molecule regulating vascular remodeling in the context of angiogenesis [90]. Del-1 was shown to interfere with LFA-1-dependent leukocyte endothelial interactions [91]. In particular, Del-1 deficiency resulted in markedly increased leukocyte adhesion and recruitment to inflamed tissues in vivo [91]. The exact mechanistic action of the inhibitory role of Del-1 in leukocyte recruitment requires detailed investigation. Soluble ICAM-5 has also been described to act as an inhibitor of LFA-1 and can decrease T lymphocyte and microglia activation in a manner opposite to the pro-inflammatory action of ICAM-1 [92]. Interestingly, the high expression of both Del-1 and ICAM-5 in the central nervous tissue may contribute to its immune privilege. Galectin-1 is another endogenous inhibitor of leukocyte recruitment. Galectin-1 inhibits T-cell rolling and adhesion to activated endothelial cells under flow conditions, whereas galectin-1-deficiency in mice induced increased homing of T lymphocytes to lymph nodes and enhanced leukocyte recruitment in the cremasteric circulation [93, 94].
Platelet-leukocyte cross-talk
Besides their well established role as the first cellular response in the coagulation cascade [95, 96], platelets are intimately involved in inflammatory reactions largely because of their direct crosstalk with leukocytes [97]. Upon vascular injury and endothelial denudation, platelets adhere and aggregate via their contacts with the free subendothelial matrix. However, platelets rapidly adhere to the activated vascular endothelium. Endothelial-adherent platelets promote further endothelial activation [98]. Indeed, platelets adhere to the vascular endothelium of the carotid artery in ApoE-deficient mice before the development of advanced atherosclerotic lesions [99-101].
The interaction of platelets with both leukocytes and endothelial cells provides an important process in inflammation [97, 102]. First, platelet adhesion on endothelial cells and the release of potent inflammatory and mitogenic substances by platelets can alter the adhesive, chemotactic and proteolytic properties of endothelial cells thereby supporting the adhesion and transmigration of leukocytes to the inflamed tissue [103, 104]. Second, activated platelets release a variety of growth factors, inflammatory cytokines and chemokines into their microenvironment that can further directly stimulate leukocytes [102]. For instance, platelets are a major source for the chemokine stromal cell-derived factor-1 (SDF-1) [105, 106], which supports leukocyte integrin activation and thereby primary adhesion of circulating leukocytes to the vascular endothelium [107]. Third, platelets can directly interact with leukocytes; the platelet-leukocyte/monocyte aggregates have been implicated in atherosclerotic lesion formation [99, 108, 109]. The platelet receptors P-selectin, GPIb and glycoprotein IIb/IIIa contribute substantially to these inflammatory processes in inflammation and atherosclerosis [99, 108-111]. Fourth, via their direct interaction with both endothelial cells and leukocytes, platelets can serve as a bridge to promote leukocyte adhesion to the vascular wall [68, 99, 110, 112]. The mechanisms involved in the crosstalk between platelets and leukocytes are multiple. Platelet-leukocyte interactions can be mediated by both selectin-dependent and integrin-dependent adhesive interactions. In particular, P-selectin on platelets interacts with PSGL-1 on leukocytes [110, 113, 114]. A central leukocyte receptor mediating adhesion to platelets is the integrin Mac-1 [115, 116]. Mac-1 can interact with several platelets receptors. For example the interaction between Mac-1 and glycoprotein Ib (GPIb) on platelets can mediate adhesive interactions between leukocytes and platelets under both high and low shear stress conditions [68, 115, 116]. Inhibition of the Mac-1/GPIb interaction has been implicated as a therapeutic target in several inflammatory diseases [115-119]. Another major ligand for leukocyte Mac-1 is platelet JAM-C, promoting recruitment of leukocytes and dendritic cells [67, 68]. Platelet ICAM-2 as well as fibrinogen bound onto platelet glycoprotein IIb/IIIa (αIIbβ3-integrin) may also serve as binding sites for leukocyte Mac-1, thereby modulating the recruitment of leukocytes to sites of inflammation by platelets [120, 121].
The relevance of leukocyte/platelet interactions is not restricted to chronic inflammatory disease, but is important in a variety of processes in immunity, including the immune response to bacterial infections. For instance, during the course of infections microbial contents trigger immune-mediated platelet activation and thrombus formation resulting in a proinflammatory and procoagulatory state of the infected tissue [122-124]. In an in vivo model of sepsis, Clark et al. demonstrated that platelets stimulate the formation of extracellular traps by neutrophils that can engulf bacteria in the septic blood [125]. Platelets can also contribute to cytotoxic T-lymphocyte (CTL) mediated liver immunopathology independently of their procoagulant function [126]. In this study, platelet depletion reduced accumulation of virus specific CTLs in mouse models of acute viral hepatitis and subsequently liver damage [126]. On the other hand, platelets and their released growth factors are important for tissue regeneration [127, 128]. Using a mouse model of liver regeneration it was shown that platelet-derived serotonin is centrally involved in the initiation of liver regeneration [128].
Conclusions
In summary, the involvement of leukocytes in local or general inflammatory process is self-evident. During the last decades it has become clear that leukocyte recruitment involves a multistep cascade of adhesive events. Improved imaging techniques will delineate the importance of the different adhesive interactions for tissue-specific and disease-specific inflammatory cell recruitment. Moreover, the role of other non-classical inflammatory cells such as platelets in inflammatory processes is increasingly elucidated, which results in a more comprehensive and thorough understanding of inflammation. Given the major importance of inflammatory processes in infectious, inflammatory and autoimmune diseases, the detailed understanding of the leukocyte recruitment cascade is an important prerequisite for developing targeted therapeutic approaches in the aforementioned pathologies.
Figure 1.
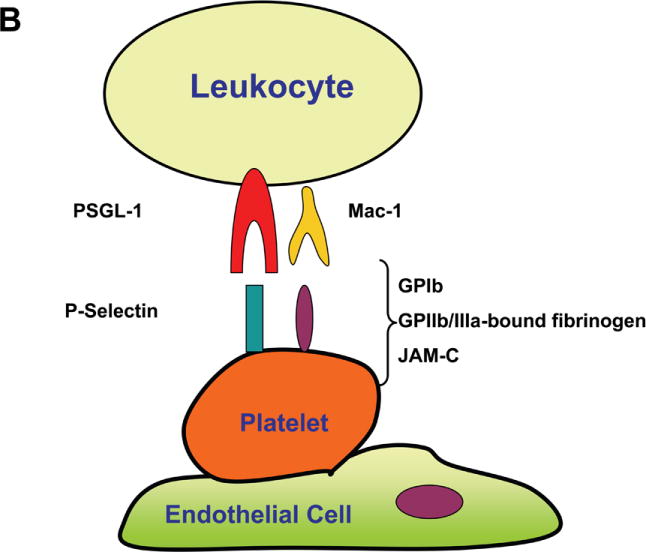
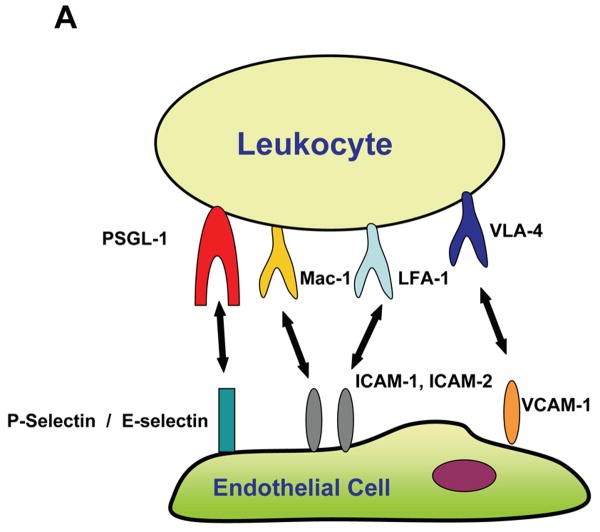
Recruitment of leukocytes to sites of inflammation depends on adhesive interactions between leukocytes and endothelial cells or endothelial cell-bound platelets.
(A) During the course of tissue inflammation, adhesive interactions between leukocytes and the endothelium include: (i) The initial rolling, which is the loose contact of the leukocyte with the endothelium, predominantly mediated by the binding of leukocyte PSGL-1 to endothelial P- and E-selectins, and (ii) The firm adhesion of leukocytes on the endothelium, which is mediated by interactions of β2-integrins such as Mac-1 and LFA-1 with the endothelial counter-receptors of the ICAM family, as well as the by the interaction of the β1-integrin VLA-4 to endothelial VCAM-1.
(B) Leukocyte recruitment can also be promoted by endothelial-adherent platelets. In this scenario, platelets can serve as a bridge between leukocytes and the endothelium. The leukocyte/platelet interaction can be mediated by leukocyte PSGL-1 binding to P-Selectin expressed on platelets, as well as by the binding of β2-integrin Mac-1 to its multiple ligands / counter-receptors on platelets such as GPIb, GPIIb/IIIa-bound fibrinogen or JAM-C.
Table 1.
Players in leukocyte extravasation
| Adhesion Molecule | Synonyms | Binding partner | General Role | Reference |
|---|---|---|---|---|
| α4β1 | VLA-4, CD49d/CD29 | VCAM-1, fibronectin | Adhesion | [129] |
| α4β7 | MAdCAM-1 | Adhesion | [130] | |
| αLβ2 | LFA-1, CD11a/CD18 | ICAM-1,-2,-3, JAM-A | Adhesion, Slow Rolling | [65, 131-133] |
| αMβ2 | Mac-1, CD11b/CD18, CR3 | iC3b, ICAM-1,-2, heparin, fibrinogen, vitronectin, kininogen, JAM-C, RAGE | Adhesion | [68, 119, 134-139] |
| αXβ2 | p150.95, CD11c/CD18 | iC3b, fibrinogen, JAM-C | [140] | |
| αDβ2 | CD11d/CD18 | ICAM-3, Fibrinogen, vitronectin | Adhesion | [141, 142] |
| JAM-A | JAM-A, LFA-1 | Adhesion, Transmigration | [62, 65] | |
| JAM-B | VLA-4, JAM-B, JAM-C | [66, 143] | ||
| JAM-C | JAM-B, JAM-C, Mac-1 | Adhesion, Transmigration | [62, 143] | |
| P-Selectin | PSGL-1, Sialyl-Lewisx | Cell Rolling | [144, 145, 146] | |
| E-Selectin | Sialyl-Lewisx | Cell Rolling | [147] | |
| L-Selectin | CD34, MAdCAM-1 | Cell Rolling | [148, 149] | |
| CD31 | PECAM-1 | CD31 | Transmigration | [77] |
| CD99 | CD99 | Transmigration | [82] | |
| ESAM | ESAM | Transmigration | [23] |
Acknowledgments
The work was supported by the NIH Intramural Research Program, National Cancer Institute and the German Academy of Sciences (Leopoldina).
References
- 1.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–73. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 3.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 4.Orlova VV, Chavakis T. Regulation of vascular endothelial permeability by junctional adhesion molecules (JAM) Thromb Haemost. 2007;98:327–32. [PubMed] [Google Scholar]
- 5.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 6.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–47. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 7.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 8.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–44. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 9.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 10.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–96. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 11.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 12.Vestweber D. Selectins: cell surface lectins which mediate the binding of leukocytes to endothelial cells. Semin Cell Biol. 1992;3:211–20. doi: 10.1016/s1043-4682(10)80017-0. [DOI] [PubMed] [Google Scholar]
- 13.Ley K, Gaehtgens P, Fennie C, Singer MS, Lasky LA, Rosen SD. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–5. [PubMed] [Google Scholar]
- 14.Nolte D, Schmid P, Jager U, Botzlar A, Roesken F, Hecht R, Uhl E, Messmer K, Vestweber D. Leukocyte rolling in venules of striated muscle and skin is mediated by P-selectin, not by L-selectin. Am J Physiol. 1994;267:1637–42. doi: 10.1152/ajpheart.1994.267.4.H1637. [DOI] [PubMed] [Google Scholar]
- 15.Steegmaier M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373:615–20. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- 16.Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994;91:7390–7. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 18.Gahmberg CG, Valmu L, Fagerholm S, Kotovuori P, Ihanus E, Tian L, Pessa-Morikawa T. Leukocyte integrins and inflammation. Cell Mol Life Sci. 1998;54:549–55. doi: 10.1007/s000180050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavakis T, Kanse SM, May AE, Preissner KT. Haemostatic factors occupy new territory: the role of the urokinase receptor system and kininogen in inflammation. Biochem Soc Trans. 2002;30:168–73. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha}L{beta}2. J Cell Biol. 2004;167:1241–53. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 22.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–34. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 23.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, Krombach F, Vestweber D. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203:1671–7. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldsmith HL, Spain S. Margination of leukocytes in blood flow through small tubes. Microvasc Res. 1984;27:204–22. doi: 10.1016/0026-2862(84)90054-2. [DOI] [PubMed] [Google Scholar]
- 25.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87. [PubMed] [Google Scholar]
- 26.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991;88:7538–42. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–75. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangelder GJ, Arfors KE. Inhibition of leukocyte rolling in venules by protamine and sulfated polysaccharides. Blood. 1991;77:1565–71. [PubMed] [Google Scholar]
- 29.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 30.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–103. [PubMed] [Google Scholar]
- 31.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–83. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205:2339–47. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etzioni A, Frydman M, Pollack S, Avidor I, Phillips ML, Paulson JC, Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med. 1992;327:1789–92. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- 34.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 35.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 36.Colditz IG, Schneider MA, Pruenster M, Rot A. Chemokines at large: in-vivo mechanisms of their transport, presentation and clearance. Thromb Haemost. 2007;97:688–93. [PubMed] [Google Scholar]
- 37.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–4. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 38.Arfors KE, Lundberg C, Lindbom L, Lundberg K, Beatty PG, Harlan JM. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–40. [PubMed] [Google Scholar]
- 39.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–94. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50:193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 41.Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O'Brien WE, Beaudet AL. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–8. [PubMed] [Google Scholar]
- 42.Basit A, Reutershan J, Morris MA, Solga M, Rose CE, Jr, Ley K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am J Physiol Lung Cell Mol Physiol. 2006;291:200–7. doi: 10.1152/ajplung.00346.2005. [DOI] [PubMed] [Google Scholar]
- 43.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–38. [PubMed] [Google Scholar]
- 44.Hogg N, Henderson R, Leitinger B, McDowall A, Porter J, Stanley P. Mechanisms contributing to the activity of integrins on leukocytes. Immunol Rev. 2002;186:164–71. doi: 10.1034/j.1600-065x.2002.18614.x. [DOI] [PubMed] [Google Scholar]
- 45.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–54. [PubMed] [Google Scholar]
- 46.Lucas R, Lou J, Morel DR, Ricou B, Suter PM, Grau GE. TNF receptors in the microvascular pathology of acute respiratory distress syndrome and cerebral malaria. J Leukoc Biol. 1997;61:551–8. doi: 10.1002/jlb.61.5.551. [DOI] [PubMed] [Google Scholar]
- 47.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz BR, Wayner EA, Carlos TM, Ochs HD, Harlan JM. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990;85:2019–22. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–56. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Dustin ML, Bivona TG, Philips MR. Membranes as messengers in T cell adhesion signaling. Nat Immunol. 2004;5:363–72. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- 51.Bazzoni G, Hemler ME. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci. 1998;23:30–4. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 52.Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr Opin Cell Biol. 2008;20:533–40. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blum MS, Toninelli E, Anderson JM, Balda MS, Zhou J, O'Donnell L, Pardi R, Bender JR. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol. 1997;273:286–94. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- 54.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol. 1998;274:810–9. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
- 55.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 56.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2008;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110:583–8. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 58.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–30. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 59.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–12. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 60.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 61.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–64. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 62.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 63.Santoso S, Orlova VV, Song K, Sachs UJ, Andrei-Selmer CL, Chavakis T. The homophilic binding of junctional adhesion molecule-C mediates tumor cell-endothelial cell interactions. J Biol Chem. 2005;280:36326–33. doi: 10.1074/jbc.M505059200. [DOI] [PubMed] [Google Scholar]
- 64.Kostrewa D, Brockhaus M, D'Arcy A, Dale GE, Nelboeck P, Schmid G, Mueller F, Bazzoni G, Dejana E, Bartfai T, Winkler FK, Hennig M. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. EMBO J. 2001;20:4391–8. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–8. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–92. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 67.Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, Wendel HP, Aebert H, Roecken M, Seizer P, Santoso S, Wesselborg S, Brossart P, Gawaz M. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–70. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 68.Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–91. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Del MA, De LA, Martin-Padura I, Brockhaus M, Bartfai T, Fruscella P, Adorini L, Martino G, Furlan R, De Simoni MG, Dejana E. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM) J Exp Med. 1999;190:1351–6. doi: 10.1084/jem.190.9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corada M, Chimenti S, Cera MR, Vinci M, Salio M, Fiordaliso F, De AN, Villa A, Bossi M, Staszewsky LI, Vecchi A, Parazzoli D, Motoike T, Latini R, Dejana E. Junctional adhesion molecule-A-deficient polymorphonuclear cells show reduced diapedesis in peritonitis and heart ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2005;102:10634–9. doi: 10.1073/pnas.0500147102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khandoga A, Kessler JS, Meissner H, Hanschen M, Corada M, Motoike T, Enders G, Dejana E, Krombach F. Junctional adhesion molecule-A deficiency increases hepatic ischemia-reperfusion injury despite reduction of neutrophil transendothelial migration. Blood. 2005;106:725–33. doi: 10.1182/blood-2004-11-4416. [DOI] [PubMed] [Google Scholar]
- 72.Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, Nawroth PP, Preissner KT, Santoso S. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–8. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- 73.Aurrand-Lions M, Lamagna C, Dangerfield JP, Wang S, Herrera P, Nourshargh S, Imhof BA. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J Immunol. 2005;174:6406–15. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 74.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–77. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 75.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703–14. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580–2. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 77.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–60. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–53. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 79.Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–60. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- 80.Sachs UJ, ndrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, Santoso S. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31) J Biol Chem. 2007;282:23603–12. doi: 10.1074/jbc.M701120200. [DOI] [PubMed] [Google Scholar]
- 81.Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood. 2004;104:3205–13. doi: 10.1182/blood-2004-03-1184. [DOI] [PubMed] [Google Scholar]
- 82.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–50. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 83.Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–80. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–88. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol. 2006;177:6440–9. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- 86.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 87.Parry RV, Riley JL, Ward SG. Signalling to suit function: tailoring phosphoinositide 3-kinase during T-cell activation. Trends Immunol. 2007;28:161–8. doi: 10.1016/j.it.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 89.Ho HK, Jang JJ, Kaji S, Spektor G, Fong A, Yang P, Hu BS, Schatzman R, Quertermous T, Cooke JP. Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: its role in ischemia. Circulation. 2004;109:1314–9. doi: 10.1161/01.CIR.0000118465.36018.2D. [DOI] [PubMed] [Google Scholar]
- 90.Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, Coleman M, Boudreau N, Varner JA. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112:30–41. doi: 10.1172/JCI17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, Ballantyne CM, Constant SL, Aird WC, Papayannopoulou T, Gahmberg CG, Udey MC, Vajkoczy P, Quertermous T, Dimmeler S, Weber C, Chavakis T. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–4. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian L, Lappalainen J, Autero M, Hanninen S, Rauvala H, Gahmberg CG. Shedded neuronal ICAM-5 suppresses T-cell activation. Blood. 2008;111:3615–25. doi: 10.1182/blood-2007-09-111179. [DOI] [PubMed] [Google Scholar]
- 93.Cooper D, Norling LV, Perretti M. Novel insights into the inhibitory effects of Galectin-1 on neutrophil recruitment under flow. J Leukoc Biol. 2008;83:1459–66. doi: 10.1189/jlb.1207831. [DOI] [PubMed] [Google Scholar]
- 94.Norling LV, Sampaio AL, Cooper D, Perretti M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. FASEB J. 2008;22:682–90. doi: 10.1096/fj.07-9268com. [DOI] [PubMed] [Google Scholar]
- 95.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–34. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 96.Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100:979–91. doi: 10.1161/01.RES.0000261936.85776.5f. [DOI] [PubMed] [Google Scholar]
- 97.von HP, Koenen RR, Weber C. Platelet-mediated enhancement of leukocyte adhesion. Microcirculation. 2009;16:84–96. doi: 10.1080/10739680802564787. [DOI] [PubMed] [Google Scholar]
- 98.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–84. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–96. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jorgensen L. The role of platelets in the initial stages of atherosclerosis. J Thromb Haemost. 2006;4:1443–9. doi: 10.1111/j.1538-7836.2006.02006.x. [DOI] [PubMed] [Google Scholar]
- 101.Theilmeier G, Michiels C, Spaepen E, Vreys I, Collen D, Vermylen J, Hoylaerts MF. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99:4486–93. doi: 10.1182/blood.v99.12.4486. [DOI] [PubMed] [Google Scholar]
- 102.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 103.Gawaz M, Brand K, Dickfeld T, Pogatsa-Murray G, Page S, Bogner C, Koch W, Schomig A, Neumann F. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148:75–85. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 104.Gawaz M. Do platelets trigger atherosclerosis? Thromb Haemost. 2003;90:971–2. [PubMed] [Google Scholar]
- 105.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1{alpha} and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–33. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stellos K, Bigalke B, Langer H, Geisler T, Schad A, Kogel A, Pfaff F, Stakos D, Seizer P, Muller I, Htun P, Lindemann S, Gawaz M. Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells. Eur Heart J. 2009;30:584–93. doi: 10.1093/eurheartj/ehn566. [DOI] [PubMed] [Google Scholar]
- 107.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Massberg S, Schürzinger K, Lorenz M, Konrad I, Plesnila N, Kennerknecht E, Rudelius M, Sauer S, Kremmer E, Emambokus N, Frampton J, Gawaz M. Reduced platelet adhesion, attenuated atherosclerosis and increased resistence to focal cerebral ischemia in mice lacking GPIIb integrin. Circulation. 2005;112:1180–8. doi: 10.1161/CIRCULATIONAHA.105.539221. [DOI] [PubMed] [Google Scholar]
- 109.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 110.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–81. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–6. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 112.Gawaz M, Stellos K, Langer HF. Platelets modulate atherogenesis and progression of atherosclerotic plaques via interaction with progenitor and dendritic cells. J Thromb Haemost. 2008;6:235–42. doi: 10.1111/j.1538-7836.2008.02867.x. [DOI] [PubMed] [Google Scholar]
- 113.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–56. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Larsen E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, Wagner DD, Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–12. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 115.Ehlers R, Ustinov V, Chen Z, Zhang X, Rao R, Luscinskas FW, Lopez J, Plow E, Simon DI. Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. J Exp Med. 2003;198:1077–88. doi: 10.1084/jem.20022181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, Lopez JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimizu K, Libby P, Shubiki R, Sakuma M, Wang Y, Asano K, Mitchell RN, Simon DI. Leukocyte integrin Mac-1 promotes acute cardiac allograft rejection. Circulation. 2008;117:1997–2008. doi: 10.1161/CIRCULATIONAHA.107.724310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, Zago AC, Lopez J, Andre P, Plow E, Simon DI. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 119.Chavakis T, Santoso S, Clemetson KJ, Sachs UJ, Isordia-Salas I, Pixley RA, Nawroth PP, Colman RW, Preissner KT. High molecular weight kininogen regulates platelet-leukocyte interactions by bridging Mac-1 and glycoprotein Ib. J Biol Chem. 2003;278:45375–81. doi: 10.1074/jbc.M304344200. [DOI] [PubMed] [Google Scholar]
- 120.Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest. 1997;100:2085–93. doi: 10.1172/JCI119742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J Clin Invest. 1994;94:1243–51. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shannon O, Hertzen E, Norrby-Teglund A, Morgelin M, Sjobring U, Bjorck L. Severe streptococcal infection is associated with M protein-induced platelet activation and thrombus formation. Mol Microbiol. 2007;65:1147–57. doi: 10.1111/j.1365-2958.2007.05841.x. [DOI] [PubMed] [Google Scholar]
- 123.Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–57. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 124.Kalsch T, Elmas E, Nguyen XD, Suvajac N, Kluter H, Borggrefe M, Dempfle CE. Endotoxin-induced effects on platelets and monocytes in an in vivo model of inflammation. Basic Res Cardiol. 2007;102:460–6. doi: 10.1007/s00395-007-0667-y. [DOI] [PubMed] [Google Scholar]
- 125.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, len-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 126.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–9. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Langer HF, Gawaz M. Platelets in regenerative medicine. Basic Res Cardiol. 2008;103:299–307. doi: 10.1007/s00395-008-0721-4. [DOI] [PubMed] [Google Scholar]
- 128.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 129.Shimizu Y, Newman W, Gopal TV, Horgan KJ, Graber N, Beall LD, van Seventer GA, Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991;113:1203–12. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 131.Dustin ML, Springer TA. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988;107:321–31. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Staunton DE, Dustin ML, Springer TA. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61–4. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- 133.de Fougerolles AR, Springer TA. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185–90. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–17. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beller DI, Springer TA, Schreiber RD. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982;156:1000–9. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Diamond MS, Alon R, Parkos CA, Quinn MT, Springer TA. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD1) J Cell Biol. 1995;130:1473–82. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Altieri DC, Agbanyo FR, Plescia J, Ginsberg MH, Edgington TS, Plow EF. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18) J Biol Chem. 1990;265:12119–22. [PubMed] [Google Scholar]
- 138.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–15. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kanse SM, Matz RL, Preissner KT, Peter K. Promotion of leukocyte adhesion by a novel interaction between vitronectin and the beta2 integrin Mac-1 (alphaMbeta2, CD11b/CD18) Arterioscler Thromb Vasc Biol. 2004;24:2251–6. doi: 10.1161/01.ATV.0000146529.68729.8b. [DOI] [PubMed] [Google Scholar]
- 140.Bilsland CA, Diamond MS, Springer TA. The leukocyte integrin p150,95 (CD11c/CD18) as a receptor for iC3b. Activation by a heterologous beta subunit and localization of a ligand recognition site to the I domain. J Immunol. 1994;152:4582–9. [PubMed] [Google Scholar]
- 141.Van der Vieren M, Le Trong H, Wood CL, Moore PF, St JT, Staunton DE, Gallatin WM. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity. 1995;3:683–90. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 142.Yakubenko VP, Yadav SP, Ugarova TP. Integrin alphaDbeta2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties. Blood. 2006;107:1643–50. doi: 10.1182/blood-2005-06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arrate MP, Rodriguez JM, Tran TM, Brock TA, Cunningham SA. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J Biol Chem. 2001;276:45826–32. doi: 10.1074/jbc.M105972200. [DOI] [PubMed] [Google Scholar]
- 144.Polley MJ, Phillips ML, Wayner E, Nudelman E, Singhal AK, Hakomori S, Paulson JC. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991;88:6224–8. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–86. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 146.Miner JJ, Xia L, Yago T, Kappelmayer J, Liu Z, Klopocki AG, Shao B, McDaniel JM, Setiadi H, Schmidtke DW, McEver RP. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112:2035–45. doi: 10.1182/blood-2008-04-149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250:1130–2. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 148.Baumheter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–8. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- 149.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–8. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]