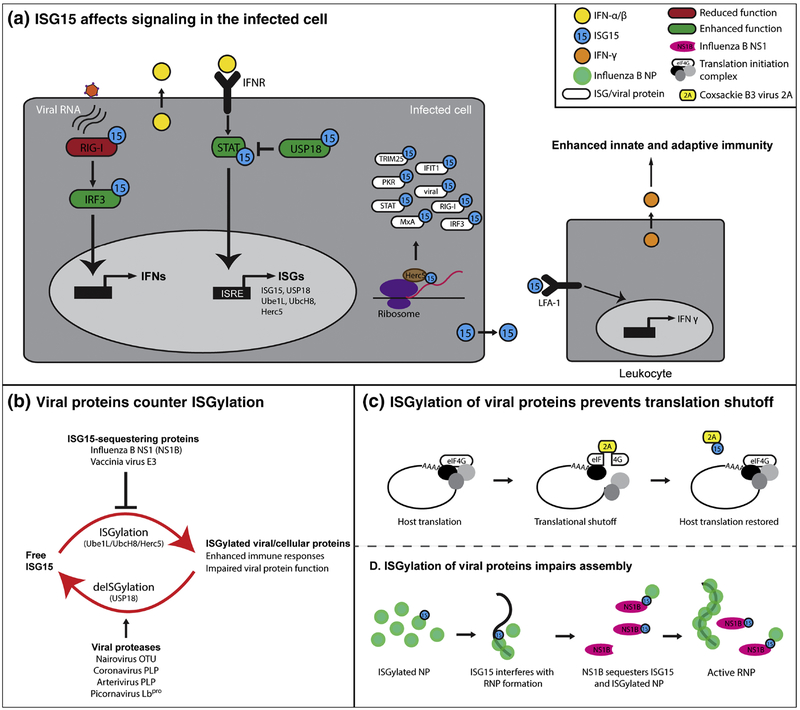
Figure 1:
ISG15 is conjugated to a wide range of viral and cellular proteins, influencing immune responses. (A) The infected host cell senses viral RNA via RIG-I, which induces signaling leading to the expression and secretion of type I IFN. Type I interferon binding to IFN receptor (IFNR) result in the expression of IFN-stimulated genes (ISGs), including ISG15 and its conjugating enzymes (Ube1L, UbcH8 and Herc5). Herc5 association to ribosomes result in the ISGylation of newly synthesized host and viral proteins including several ISGs (white). ISG15 conjugation can inhibit (red) or enhance (green) the activation state of innate immune signaling proteins. USP18 is a negative regulator of IFNR signaling by limiting STAT activation. USP18 protected from degradation by ISG15 binding, thereby enhancing the inhibition of IFNR signaling. Extracellular ISG15 binds to LFA-1 receptors of leukocytes and induces the expression and secretion of IFN-ɣ. (B) Viral proteins can counter ISGylation by sequestering ISG15/ISGylated proteins, or by deconjugating ISG15. (C) The 2A protease of Coxsackie B3 virus induces host translational shutoff by cleaving eIF4G. ISGylation of 2A restores host translation by preventing eIF4G cleavage. (D) ISGylation of viral proteins interferes with virus replication. ISGylation of Influenza B virus NP reduces the oligomerization and formation of viral ribonucleoprotein complexes (RNPs). NS1B counters ISGylation by sequestering ISG15 and ISGylated NP.