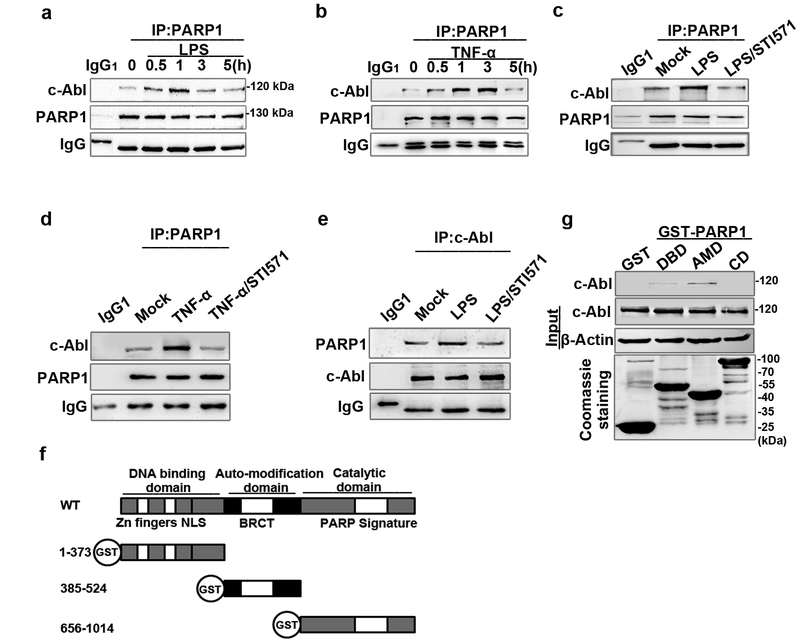
Figure 2. c-Abl interacts with PARP1 in response to exposure to inflammatory agents.
(a and b) Exposure to inflammatory agents increases the association of c-Abl with PARP1. RAW 264.7 cells were exposed to LPS (a) or TNF-α (b) for various lengths of time. Whole-cell extracts (WEs) were prepared and immuno-precipitates were obtained using Ab recognizing PARP1. The association of c-Abl with PARP1 was detected by western blotting. (c, d and e) STI571 blocked the association of c-Abl with PARP1. RAW 264.7 cells were mock-treated or exposed to LPS (±STI571) for 1 h (c and e) and TNF-α (±STI571) for 3 h (d). WEs were prepared and immuno-precipitates were obtained using Ab recognizing PARP1 or c-Abl. The blockage in association of c-Abl with PARP1 or vice versa was detected by western blotting. (f) Diagram of the domains in PARP1 and the schematics of the GST-PARP1 domains expression plasmids. (g) Auto modification domain (AMD) mediates the association of PARP1 with c-Abl. GST and GST-PARP1 domains were incubated with equal amounts of WEs from LPS-treated cells. Levels of pulled down c-Abl were detected by western blotting. Similar results were obtained from at least three independent experiments.