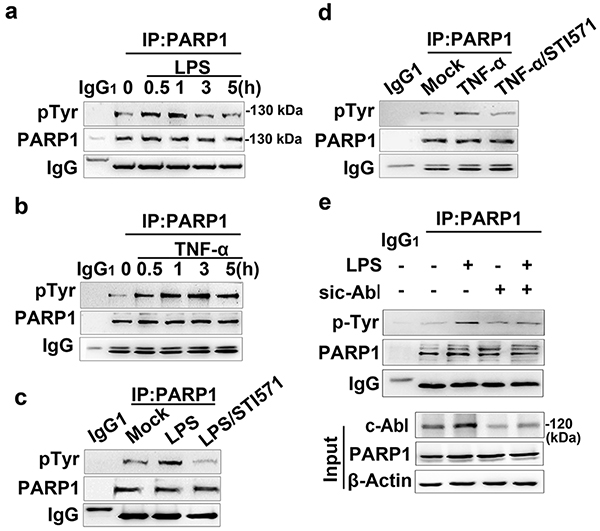
Figure 4. c-Abl is required for tyrosine phosphorylation of PARP1 in response to inflammatory agents.
(a and b) Exposure of inflammatory agents increases the levels of tyrosine phosphorylation (pTyr) of PARP1. RAW 264.7 cells were exposed to LPS (a) or TNF-α (b) for various lengths of time. Whole-cell extracts (WEs) were prepared and immuno-precipitates were obtained using Ab recognizing PARP1. The levels of pTyr of PARP1 were detected by western blotting. (c and d) Inhibition of c-Abl activity eliminates the inflammatory agent-induced increase in pTyr of PARP1. RAW 264.7 cells were mock-treated or exposed to LPS (±STI571) for 1 h (c), or TNF-α exposed (±STI571) for 3 h (d). WEs were prepared and immuno-precipitates were obtained using Ab recognizing PARP1. The levels of pTyr of PARP1 were detected by western blotting. (e) Small interfering RNA of c-Abl interferes with LPS-induced PARP1 tyrosine phosphorylation. RAW 264.7 cells were transfected with siRNA targeting c-Abl or a control for 48 h, and then challenged with LPS for 1 h. WEs were prepared and immuno-precipitates were obtained using Ab recognizing PARP1. The levels of pTyr of PARP1 in the presence or absence of c-Abl were detected by western blotting. Similar results were obtained from at least three independent experiments.