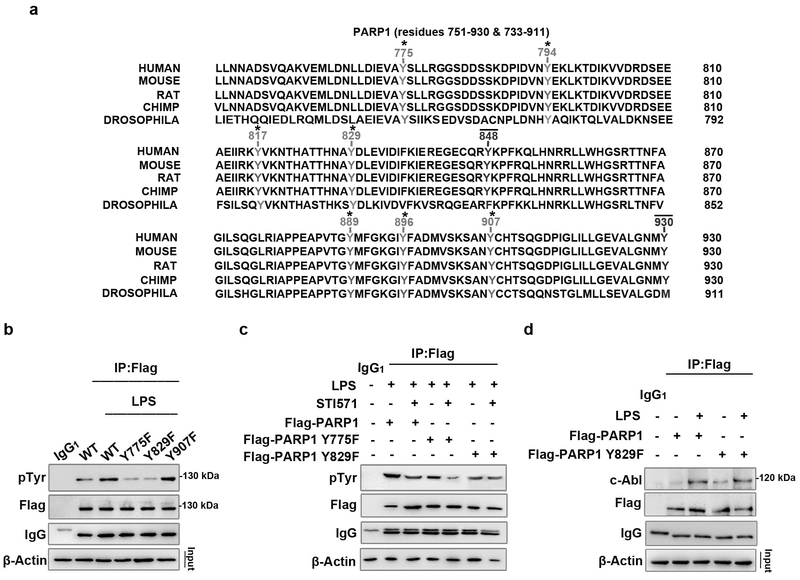
Figure 5. PARP1 Y829 might be the site phosphorylated by c-Abl in response to LPS stimulation.
(a) Conservative comparison of tyrosine residues of human PARP1 in segment 751–930 with those relevant in other species through drosophila to primate. Tyrosine residues that are conserved across all sequences are highlighted with (*) sign and tyrosine residues that are not conserved with (−) sign. (b) Y775 and Y829 sites of PARP1 undergo phosphorylation in LPS-exposed cells. Human Embryonic Kidney 293/hTLR4A-MD2-CD14 (HEK 293) cells were transfected with wild type (WT) Flag-PARP1 as well as Y775F, Y829F and Y907F mutant plasmids, and then challenged with LPS for 1 h. Immuno-precipitates were prepared using flag Ab and then subjected to western blotting to detect phosphorylation level. (c) The Y829 site of PARP1 might be phosphorylated by c-Abl in response to LPS exposure. Wild type Flag-PARP1, as well as both Y775F and Y829F mutants were transfected in HEK 293 cells, then the cells were stimulated with LPS (±STI571) for 1 h. Immunoprecipitates were prepared using Flag Ab and then subjected to western blotting to detect phosphorylation levels. (d) Y829F mutation does not affect the interaction of c-Abl and PARP1. HEK 293 cells were transfected with WT Flag-PARP1 and Y829F mutant plasmids, and then challenged with LPS or left untreated for 1 h. Immuno-precipitates were prepared using Flag Ab and then subjected to western blotting to detect the interaction with c-Abl. Similar results were obtained from at least three independent experiments.