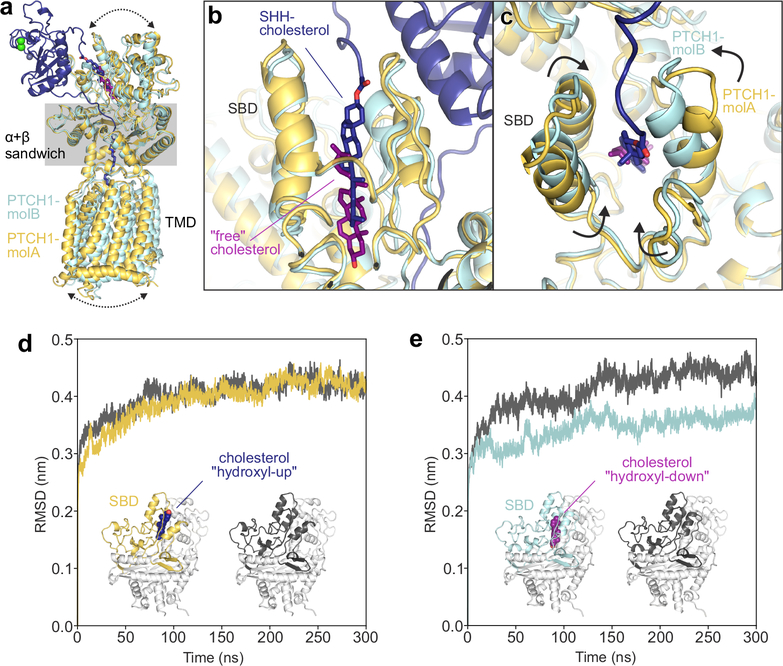
Figure 5 |. The PTCH1 sterol-binding domain (SBD) can bind cholesterol in two opposite orientations.
a-c, Comparison of PTCH1-molA (yellow) and -molB (cyan) from the rebuilt 2:1 PTCH1-pShhNc complex. a, Superposition of molA and molB templated on the α+β sandwich domain. Differences in the relative positions of the TMD and the upper lobes of the ECD are indicated by arrows. b, Close-up of the SBDs from the superimposed structures. PTCH1-molA binds to the ShhN-attached cholesterol (dark blue) with the esterified cholesterol positioned at the SBD entrance (“hydroxyl-up”), while the cholesterol in PTCH1-molB is buried in the SBD core in an inverted orientation (“hydroxyl-down”). c, Conformational differences at the mouth of the SBD in PTCH1-molA and -molB. d and e, Cholesterol orientation-dependent differences in the stability of the PTCH1 upper lobe in molecular dynamics simulations. Average RMSD (compared to the starting structure) of the PTCH1ECD1 upper lobe across five independent atomistic simulations (300 ns each) of the entire PTCH1ECD from molA (d) and molB (e), with (yellow/cyan) and without (grey) bound cholesterol.