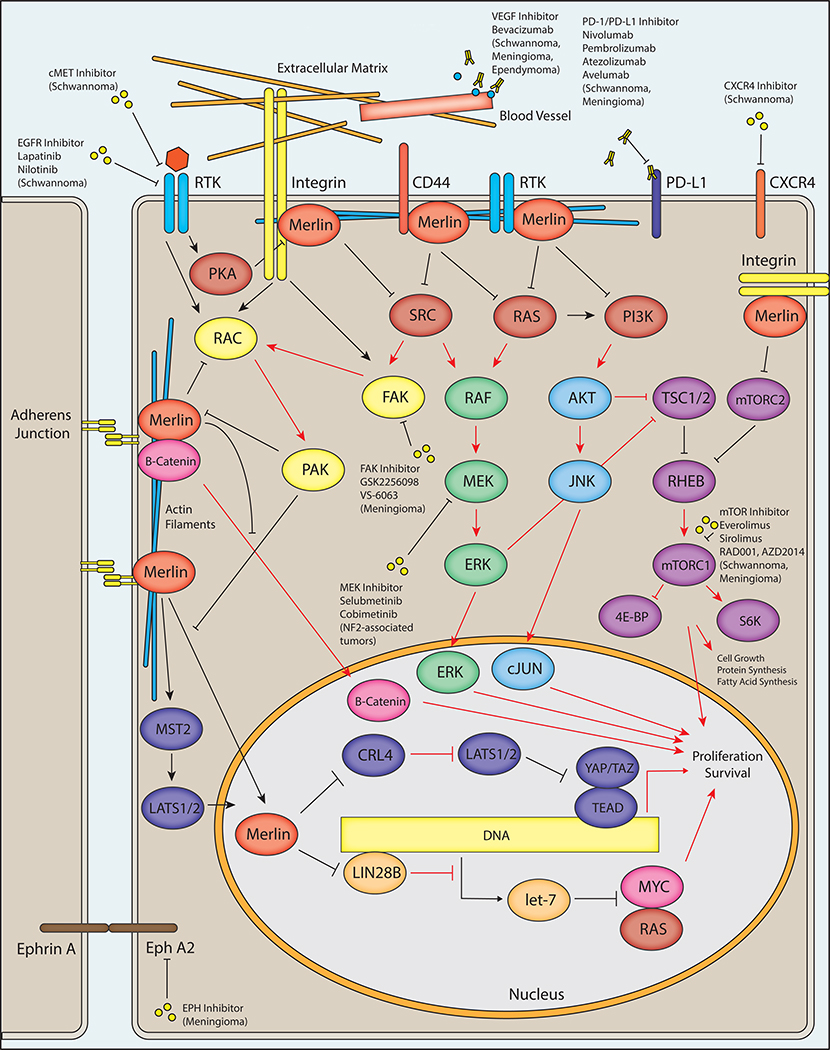
Figure 1 – NF2/Merlin Signaling and Potential Therapeutic Avenues in NF2.
A schematic diagram depicts major intracellular pathways regulated by merlin, the protein product of the NF2 gene. Merlin is associated with the plasma membrane via its N-terminal FERM domain, where it interacts with adherens junctions and numerous cell surface receptors including integrins, receptor tyrosine kinases (RTK), and CD44. The C-terminal domain of merlin interacts with cytoskeletal actin filaments. Merlin has a predominantly inhibitory activity on downstream effectors including RAS, PI3K, RAC, and SRC, which may result in reduced downstream RAF/MEF/ERK, AKT/JNK/JUN, mTORC1, FAK, and RAC signaling. Merlin may inhibit translocation of β-catenin to the nucleus, reducing the output of canonical Wnt/β-catenin signaling. Merlin also interacts with the Hippo pathway by promoting MST1/2-mediated translocation of LATS1/2 to the nucleus as well as through inhibition of CRL4DCAF1, resulting in reduced transcriptional output of YAP/TAZ and TEA domain transcription factors (TEADs). In the nucleus, merlin may also inhibit the activity of LIN28A, thereby relieving inhibition of the let-7 miRNA cluster leading to downregulation of proto-oncogenic proteins such as MYC and RAS. Loss of function of merlin via pathogenic mutations in NF2 patients results in alteration of downstream activity in each of these pathways (red arrows indicate steps with possible increased activity following loss of merlin function, as in NF2-associated tumors), potentially leading to increased cell growth, protein and fatty acid synthesis, proliferation, and survival. A variety of therapeutic avenues have been explored in NF2 patients, including inhibition of proteins regulated by merlin as well as other cellular receptors. Inhibition of the RAF/MEK/ERK pathway via small molecule MEK inhibitors is currently being explored in clinical trials for NF2-associated tumors ( NCT02639546, NCT03095248). A phase 2 clinical trial is exploring small molecule inhibition of FAK by GSK2256098 (NCT02523014/A071401) in NF2 mutant meningiomas. Small molecule mTOR inhibitors including everolimus and sirolimus have shown therapeutic promise in NF2-associated schwannomas and meningiomas, and additional compounds including RAD001 and AZD2014 are in clinical trials for the treatment of progressive/symptomatic NF2-associated meningiomas ( NCT02831257) and recurrent high-grade (WHO grade II/III) meningiomas ( NCT03071874), respectively. The VEGF inhibitor bevacizumab has shown clinical utility in NF2-associated schwannomas, meningiomas, and ependymomas. Inhibition of the tyrosine kinase receptors cMET and EGFR may provide additional therapeutic opportunities in NF2-associated meningiomas. Additional therapeutic targets have been proposed, including the immunomodulatory PD-1/PD-L1 axis, the chemokine receptor CXCR4, and Ephrin receptor A2 (EphA2).