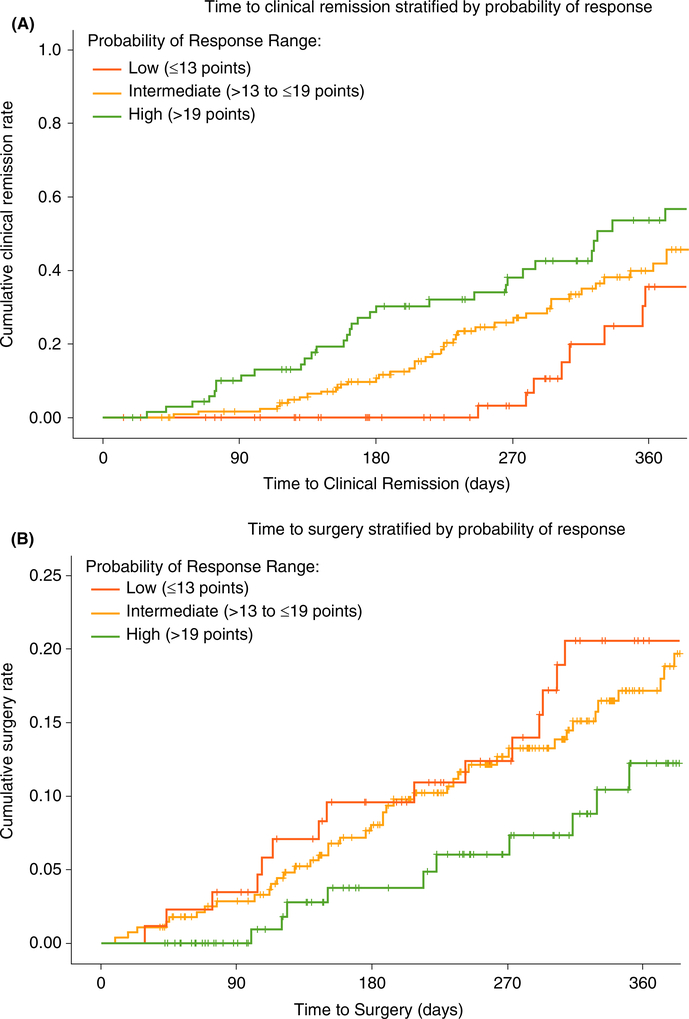
FIGURE 4.
VICTORY consortium vedolizumab-treated rates of endoscopic remission and progression to surgery stratified by CDST. A, Cumulative rates of endoscopic remission. B, Cumulative rates of progression to surgery. Low probability; ≤13 points in CDST model at baseline. Intermediate probability; >13 to ≤19 in CDST model at baseline. High probability; >19 points in CDST model at baseline. High-probability group (n = 131), intermediate-probability group (n = 281), low-probability group (n = 89). Abbreviation: CDST, clinical decision support tool. Analysis of endoscopic remission limited to those patients with follow-up endoscopic assessments (n = 326; high probability n = 84; intermediate probability n = 172; low probability n = 70). Endoscopic remission defined as absence of ulcerations. Pairwise log-rank comparisons across the three probability groups for endoscopic remission: high vs low P < 0.001; high vs intermediate P = 0.076; low vs intermediate P = 0.002. Pairwise log-rank comparisons across the three probability groups for progression to surgery: high vs low P = 0.024; high vs intermediate P = 0.076; low vs intermediate P = 0.264