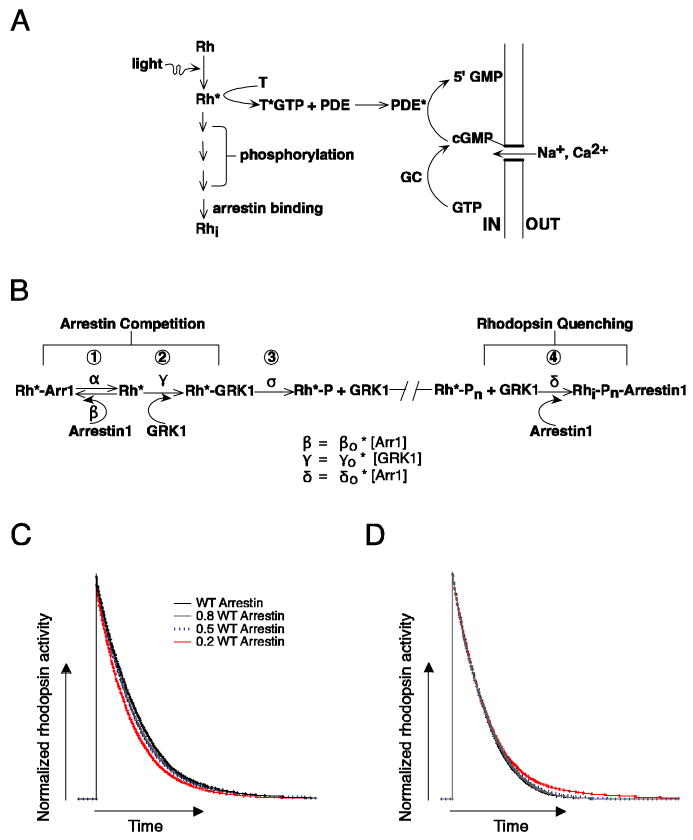
Figure 1.
Biochemical readout and control of rhodopsin activity. (A) Schematic of phototransduction cascade in mammalian rods. Rhodopsin (Rh) is activated by the absorption of a photon. Activated rhodopsin (Rh*) leads to the sequential activation of transducin (T) and phosphodiesterase (PDE). PDE* hydrolyzes cGMP and leads to the closure of cGMP-gated channels in the membrane of the rod outer segment. Inactivation of Rh* requires multiple phosphorylations of rhodopsin's C terminus by GRK1 followed by arrestin1 quenching. Guanylate cyclase (GC) maintains cytoplasmic cGMP. (B) Schematic diagram of the molecular reactions involved in the inactivation of a Rh* molecule. The duration of each rhodopsin phosphorylation event depends on the competition between arrestin1 and GRK1 binding, and the time required for phosphate attachment. Reactions 1 to 3 cycle until a sufficient number of phosphates (Pn) are incorporated to the C terminus. Wild-type mouse rhodopsin has 6 potential phosphorylation sites. The final shutoff step is provided by arrestin1. Arrestin competition (reaction 1) slows Rh* shutoff while the binding of arrestin1 to Rh*-Pn (reaction 4) terminates Rh* activity. (C) Simulation of the combined effect of arrestin competition and quenching on rhodopsin inactivation. Rhodopsin inactivation was described as a series of 6 phosphorylation steps as in 1-3 of B, followed by arrestin1 binding (see Materials and Methods for details). The rate constants β and γ in B scaled linearly with arrestin1 concentration. The net effect of decreasing arrestin1 concentration can be to shorten Rh* activity. (D) Decreasing the arrestin1 concentration would lengthen Rh* activity if arrestin's only role is to quench Rh*. Simulations were identical to C except β did not depend on arrestin1 concentration.