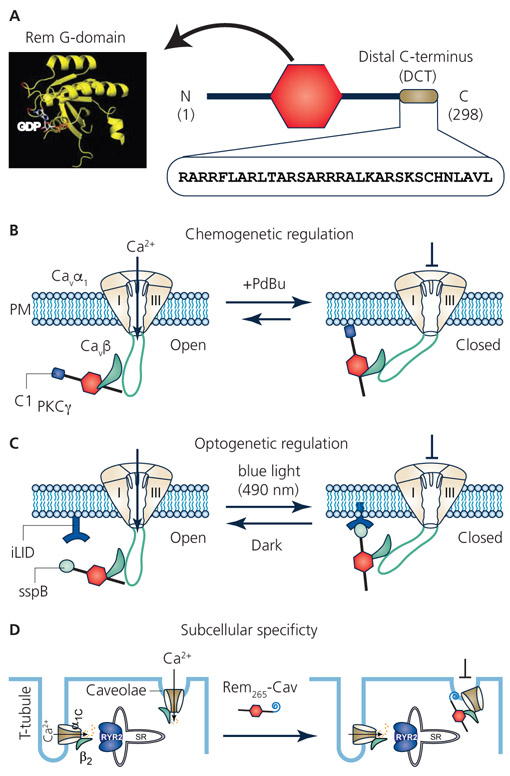
Figure 2. Replacing Rem distal C-terminus for novel spatio-temporal control of CaV channel inhibition.
A. Rem structure consists of a guanine nucleotide binding domain (G-domain) flanked by N- and C-termini. The Rem distal C-terminus (DCT), comprised of the last 32 residues of the protein, is a polybasic peptide that mediates binding to the plasma membrane and is necessary for CaV channel inhibition. B. Replacing Rem DCT with C1 domain from protein kinase C γ (C1PKCγ) enables acute recruitment of the engineered Rem to the plasma membrane with a small molecule phorbol ester, PdBu. Co-expressed CaV1/CaV2 channels are inhibited concomitantly with Rem265-C1PKCγ association with the plasma membrane. This chemo-genetic configuration provides acute temporal control over CaV channel inhibition that is slowly reversible. C. Optogenetic control of Rem inhibition of CaV channels was achieved using the photodimerizer pair, iLID (LOV2-ssrA) and sspB. The Rem DCT was replaced with sspB via varying linkers (creating optoRGK) while iLID was constitutively anchored to the plasma membrane. Exposure of cells to blue light (470 nm) enabled acute recruitment of optoRGK to the plasma membrane and inhibition of CaV1.2 channels. Both plasma membrane association of optoRGK and CaV1.2 channel inhibition were reversed in the dark. (C) Replacing Rem DCT with a caveolae-targeting peptide enabled selective inhibition of caveolae-targeted CaV1.2 channels in cardiomyocytes while sparing dyadic CaV1.2 channels that mediate cardiac excitation-contraction coupling.