Abstract
Background:
We previously found that PD-L1 expression is increased on tumor cells following vaccination treatments that lead to increased tumor-specific T cells that secrete IFNγ. Indoleamine 2,3-dioxygenase (IDO) is another IFNγ inducible gene that has potent immunosuppressive effects. There have been reports of IDO expression in prostate cancer, however it is unknown whether IDO expression might similarly increase in prostate tumors following T-cell-based immunotherapy.
Methods:
Blood samples from normal male blood donors (n=12) and patients with different stages of prostate cancer (n=89), including patients with metastatic, castration-resistant prostate cancer treated with a DNA vaccine and/or pembrolizumab, were evaluated for IDO activity by kynurenine and tryptophan levels. Metastatic tissue biopsies obtained pre- and post-treatment were evaluated for IDO expression. IDO suppression of vaccine-induced T-cell function was assessed by ELISPOT.
Results:
Overall, IDO activity was increased in patients with more advanced prostate cancer. This activity, and IDO expression as detected immunohistochemically, increased following treatment with either a DNA vaccine encoding the prostatic acid phosphatase (PAP) tumor antigen or PD-1 blockade with pembrolizumab. Increased IDO activity after treatment was associated with the absence of clinical effect, as assessed by lack of PSA decline following treatment. Increased antigen-specific T-cell response, as measured by IFNγ release, to the vaccine target antigen was detected following in vitro stimulation of peripheral blood cells with 1-methyltryptophan.
Conclusions:
These findings suggest that IDO expression is a mechanism of immune evasion used by prostate cancer, and that future clinical trials using T-cell based immune strategies might best include IDO inhibition.
Keywords: Indoleamine 2,3-dioxygenase (IDO); pTVG-HP; prostatic acid phosphatase (PAP); DNA vaccine; prostate cancer; pembrolizumab
Précis:
IDO activity is increased in patients with advanced prostate cancer and expression is augmented following immunotherapy in a subset of patients, suggesting IDO inhibition be used in combination with T-cell activating therapies for prostate cancer.
BACKGROUND:
The growth of tumors in an immune competent host suggests that mechanisms of immune avoidance are employed to evade immune-mediated destruction (1). Tumors can acquire or use many different means of immune avoidance, including 1) acquisition of immunosuppressive cell populations such as regulatory T cells, myeloid derived suppressor cells (MDSC), and tumor-associated macrophages; 2) expression of ligands such as PD-L1 that can interfere with T cell function; 3) loss of expression of immune recognition markers such as MHC class I; and 4) elaboration of cytokines and chemokines that can interfere with immune cell function and recognition. Indoleamine 2,3 dioxygenase (IDO), an INFγ-inducible tryptophan metabolizing enzyme, is one such factor produced in the tumor microenvironment and has known immunosuppressive properties (2, 3). IDO is the first and rate-limiting catabolic enzyme in the degradation pathway of tryptophan. By cleaving the aromatic indole ring of tryptophan, IDO initiates the production of a variety of tryptophan degradation products, including kynurenines. Tryptophan depletion, and the production of kynurenine and other metabolites, leads to profound immune-regulatory functions, including inhibiting lymphocyte expansion and recruitment of regulatory T cells and myeloid-derived suppressor cells to the tumor microenvironments (4–6). Expression of IDO has previously been established as one potential mechanism of resistance to CTLA-4 blockade therapy, spurring the clinical development of IDO inhibitors as potential anti-cancer therapies to combine with T-cell checkpoint inhibitors (7). However, one clinical study assessing IDO inhibition using epacadostat in combination with PD-1 blockade with pembrolizumab failed to demonstrate improvement over pembrolizumab treatment alone (8).
Prostate cancer is generally considered to be a poorly immunogenic cancer, devoid of large numbers of tumor-infiltrating lymphocytes (TIL), and containing immunosuppressive populations, including MDSC. Unlike many solid tumors, prostate cancer has been relatively refractory to treatment with CTLA-4 or PD-1/PD-L1 targeted therapies alone. On the other hand, IDO expression has been detected in murine TRAMP prostate tumors and was associated with early prostate cancer progression, suggesting that IDO might be a therapeutic target for prostate cancer (9). Likewise, IDO expression has been detected in human prostate cancers (10) and in human prostate cancer undergoing epithelial-mesenchymal transition (11). However the detection of IDO activity, at least as measured by serum kynurenine-to-tryptophan (kyn:trp) ratio, has been debated as a biomarker of prostate cancer detection or progression (12, 13). In addition, it remains unknown whether IDO expression is specifically used by prostate cancer as a mechanism of immune evasion.
We have previously demonstrated that PD-L1 expression increases on tumor cells following anti-tumor vaccination, mediated by antigen-specific cells secreting IFNγ (14). We have also found that PD-L1 expression increases on circulating human prostate cancer cells following anti-tumor vaccination targeting prostatic acid phosphatase (PAP) (15). These findings have led us to explore the combination of PD-1 blockade with anti-tumor vaccination in murine models and clinical trials (14, 16, 17). Because IDO expression is similarly induced following exposure of cells to IFNγ, we questioned whether IDO expression or activity might be induced following prostate cancer immunotherapy (18).
In this report, we used sera from patients with different stages of prostate cancer and found that IDO activity, as assessed by serum kyn:trp ratios, increased with stage of disease. This activity, and IDO expression in tumors, was markedly induced following treatment with an anti-tumor vaccine and/or PD-1 blockade. IDO activity was found to suppress the function of vaccine-induced T cells, and to be highest in patients who did not demonstrate benefit from immunotherapy. Together, these findings suggest that IDO is a mechanism of resistance to prostate cancer directed immunotherapy. Future clinical trials should explore IDO inhibition with T-cell targeted therapies.
MATERIALS AND METHODS:
Patient and Sample Populations:
Sera or plasma and peripheral blood mononuclear cells (PBMC), cryopreserved at −80°C or in liquid nitrogen, respectively, were used for these studies. These samples were collected from men without prostate cancer (n=12), patients with newly diagnosed prostate cancer (n=14), patients with biochemically recurrent (rising PSA), non-metastatic prostate cancer that were either non-castrate (n=15), or castration-resistant (n=15), and from men with castration-resistant metastatic prostate cancer (n=16).
Tryptophan and kynurenine analysis:
Tryptophan and kynurenine concentrations were measured directly in serum samples using a clinically validated LC/MS method. All analysis was performed by Worldwide Clinical Trials (Morrisville, NC) by personnel blinded to the sample source.
Enzyme-Linked Immunosorbent Assay (ELISA) for Serum IFNγ:
Sera samples were evaluated for IFNγ concentration by capture ELISA using standard methods as previously described (19). Antibodies included an anti-human IFNγ capture antibody (BD Biosciences, San Jose, CA #554550) and biotinylated anti-human IFNγ detection antibody (BD Biosciences #551221).
Immunohistochemistry:
Formalin-fixed paraffin-embedded (FFPE) tumor biopsies were stained for IDO, prostate-specific membrane antigen (PSMA), or CD163 expression using standard immunofluorescent (IF) techniques. Briefly, slides were heated at 80°C for 20 min, deparaffinizied, and antigens retrieved using DIVA Decloaker (Biocare Medical, DV2004, Pacheco, CA) at 99°C for 30 min. IDO was detected with primary antibody (Biocare Medical, ACI 3210 B) diluted 1:100 in Renoir Red diluent (Biocare Medical, PD904) followed by an AlexaFluor 488 labeled anti-mouse secondary antibody (Cell Signaling, 4408S, Danvers, MA) and subsequently mounted in ProLong Gold Antifade Reagent with DAPI (Cell Signaling, 8961S). PSMA and CD163 were detected with primary antibodies (12815S [1:100] and 934985 [1:500], Cell Signaling) diluted in Van Gogh Diluent (Biocare Medical, PD902 L) labeled with anti-rabbit AlexaFluor 555 secondary (4413S [1:500], Cell Signaling) and mounted in ProLong Gold Antifade Reagent with DAPI. For dual staining, primary and secondary antibodies were combined and co-stained in the Van Gogh diluent. Imaging was conducted on a Leica DMi8 and images processed in the Fiji package of ImageJ (20). The contrast, brightness and color balance were optimized evenly across all areas of each image and for all images.
Image Processing:
Whole FFPE tumor biopsies were stained with IDO/PSMA or IDO/CD163 and DAPI as described above. Whole section mosaic images were obtained on the Leica DMi8 at 10x (Figure S3, A–B). A threshold was then determined for the original greyscale images using the ImageJ built-in IsoData algorithm for IDO and Huang algorithm for PSMA. The threshold was applied, and the image converted into a binary mask. Because PSMA is a membrane stain, for that protein the conversion was followed by the “fills holes” ImageJ function. Once the binary images were created a selection was made using the ImageJ built-in function (edit/selection/create selection) and the area quantified using the measure function (process/measure) to give total tumor (AT) and IDO-expressing (AI) areas. To calculate the percentage of AT that overlapped with AI, the selection of IDO was applied to the mask of PSMA, filled white, and the remaining tissue area measured as described above. The process was repeated for PSMA over IDO. To calculate the IDO+ area within tumor (AT+I), the area of tumor without IDO (AT-I) was subtracted from the total area of tumor (AT). AT+I was divided by AT to give the percentage of total tumor area positive for IDO. A further description and examples of this analysis are provided in Figure S3.
ELISPOT:
ELISPOT for the measurement of IFNγ release was performed as previously described (21). For these analyses, cryopreserved PBMC were thawed, and cultured with PAP protein antigen (Fitzgerald Industries, Acton, MA), media alone, or phytohemaglutinin (PHA). Stimulations were also conducted in the presence of 2 mM 1-methyl-d1-tryptophan (1MT, R&D Systems, Minneapolis, MN). After 48 hours, plates were developed and spots enumerated using an automated ELISPOT reader (ImmunoSpot, CTL, Shaker Heights, OH).
Statistical Analysis:
Comparison of medians was made using T-tests or Wilkoxon ranked sum tests as indicated. Multiple comparisons across groups were made by Kruskal-Wallace test, with Dunn’s correction for multiple comparisons. Correlations between linear variables parameter were made using a Pearson correlation coefficient. Analyses were conducted using GraphPad Prism software (version 5.01). For all analyses, a p value ≤ 0.05 was considered statistically significant.
RESULTS:
Patients with advanced prostate cancer have higher IDO activity.
Sera samples from male volunteer blood donors without prostate cancer (n=12, median age 47, range 45–62), patients with newly diagnosed prostate cancer (n=14, median age 64, range 53–82), patients with non-metastatic, non-castrate, PSA-recurrent prostate cancer (n=15, median age 68, range 53–74), patients with non-metastatic, castration-resistant, PSA-recurrent prostate cancer (n=15, median age 73, range 60–89), and patients with castration-resistant, metastatic prostate cancer (n=16, median age 70, range 54–83) were evaluated for tryptophan and kynurenine concentrations as an assessment of IDO activity. The samples from patients with metastatic, castration-resistant prostate cancer (mCRPC) were those obtained at baseline from a clinical trial in which they subsequently received tumor-targeted vaccination and PD-1 blockade (17). As shown in Figure 1A, the kynurenine-to-tryptophan (kyn:trp) ratio was generally higher in patients with prostate cancer compared with male volunteer blood donors, and highest in patients with more advanced stage of disease. However, because prostate cancer is an age-associated disease, kyn:trp ratios were assessed with respect to age as well as the corresponding serum PSA level. As shown in Figures 1B and 1C, kyn:trp was associated with age and loosely associated with tumor volume, using serum PSA as a general assessment of tumor volume. Similar results were found for kynurenine concentrations directly (Figure S1).
Figure 1: Kynurenine:tryptophan ratios are higher in patients with advanced prostate cancer.
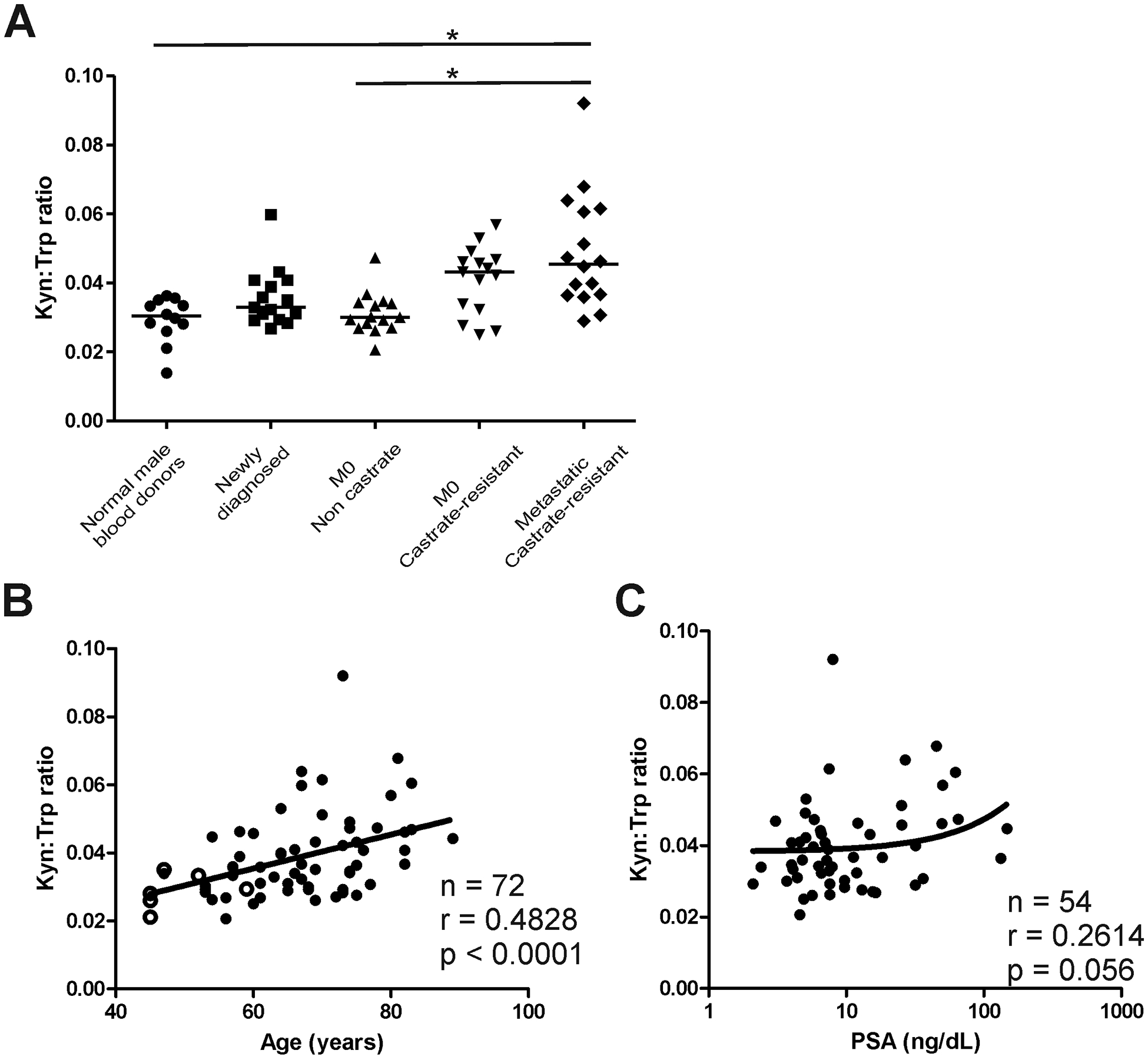
Sera or plasma samples were evaluated for kynurenine and tryptophan concentrations from normal male volunteer blood donors (n=12), patients with newly diagnosed prostate cancer pre-treatment (n=14), patients with non-castrate, PSA-recurrent non-metastatic (M0) prostate cancer (n=15), castration-resistant, M0 prostate cancer (n=15), and castration-resistant, metastatic prostate cancer (n=16). Shown are the ratios of kynurenine-to-tryptophan for each group (panel A), and overall with respect to subject age (panel B) or serum PSA for individuals with prostate cancer (panel C). Open circles in panel B are normal male blood donors. For panel A, * = p< 0.05 (Kruskal-Wallis test with Dunn’s correction for multiple comparisons). Tests of correlation with age and PSA (panels B and C) were made by Spearman test.
Patients with prostate cancer treated with an anti-tumor vaccine and/or PD-1 blockade develop increased IDO activity and expression.
We have previously demonstrated in mice that vaccination targeting a tumor-associated antigen, with induction of IFNγ-secreting T cells specific for the antigen, elicits PD-L1 expression on tumor cells (14). We similarly found that PD-L1 expression increased on circulating tumor cells following vaccination of patients with prostate cancer using either sipuleucel-T or a DNA vaccine, both targeting the PAP prostate tumor antigen (15). Because IDO is also an IFNγ-regulated gene, we questioned whether prostate cancer immunotherapy similarly elicited increases in IDO expression. We have recently reported the results of a trial in which patients with mCRPC were treated with a DNA vaccine encoding PAP alone for 12 weeks, followed by pembrolizumab over the subsequent 12 weeks, or were treated with both agents for 12 weeks (17). Sera was assessed for kynurenine and tryptophan concentrations at baseline and at 12 and 24 weeks, effectively permitting an analysis of changes in IDO activity following treatment with vaccine alone, pembrolizumab alone, or the combination. As shown in Figure 2, kyn:trp ratios generally increased pre-treatment to post-treatment over 12 weeks in patients treated with pembrolizumab alone (n=8, Figure 2A, median 0.061 to 0.066, p=0.12), vaccine alone (n=10, Figure 2B, median 0.049 to 0.053, p=0.098), and less with both concurrently (n=6, Figure 2C, median 0.038 to 0.044, p=0.26). Kyn:trp ratios increased primarily in patients who did not experience a PSA decline during the 12-week period of treatment (n=17, Figure 2E, median 0.053 to 0.061, p=0.007), compared with those who did experience a PSA decline during the 12-week period of treatment (n=7, Figure 2D, median 0.037 to 0.046, p=0.88). IFNγ concentration was also directly evaluated in sera samples. As shown in Figure 2F, IFNγ concentration was correlated to kyn:trp ratios determined from the same sera samples.
Figure 2: Patients with prostate cancer treated with an anti-tumor vaccine and/or PD-1 blockade develop increased IDO activity.
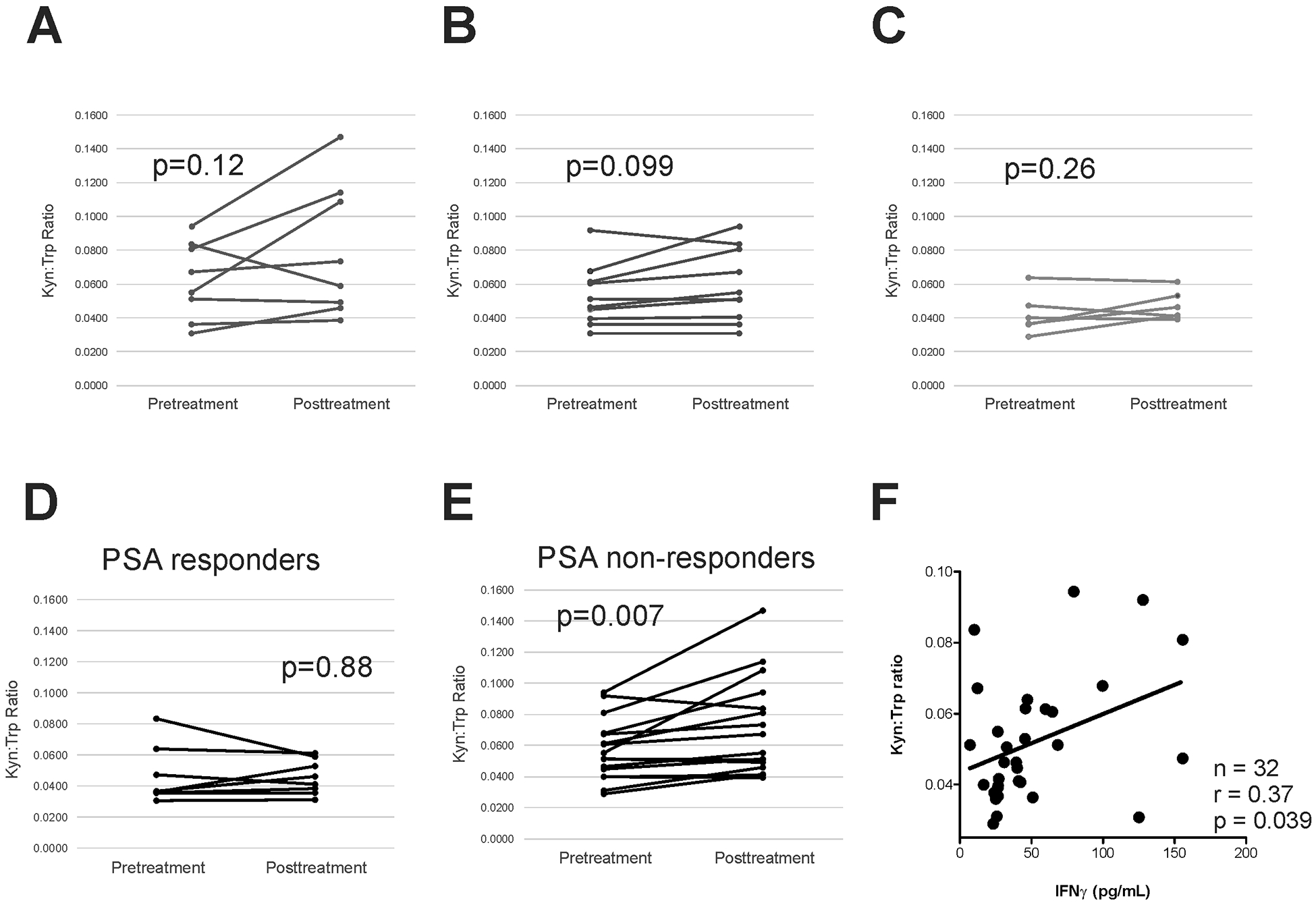
Kyn:trp ratios were evaluated in patients prior to treatment and after 12 weeks treatment with, pembrolizumab alone (n=8, panel A), pTVG-HP DNA vaccine alone (n=10, panel B), or both agents together (n=6, panel C). Kyn:trp ratios were evaluated in subsets of patients who experienced any PSA decline over the same 12-week period of treatment (n=8, panel D), and those who did not have any PSA decline over the same 12-week period of treatment (n=16, panel E). Comparisons were made with a paired student t test. Kyn:trp ratios were evaluated with respect to serum IFNγ concentration (panel F). Test of correlation was made by Spearman test.
Biopsies were collected from individual metastatic lesions in nine patients at baseline and after 12 weeks who had received either vaccine alone, or a combination of vaccine and pembrolizumab. As shown in Figure 3A and 3B (and Figure S2), IDO staining within tumors was detectable and predominantly in the extracellular matrix in close proximity to IDO+ cells. Quantification of IDO staining within tumor regions demonstrated a significant increase after treatment in matched biopsies obtained from 8 patients (p=0.02, Wilcoxon signed rank test). The majority of cells staining positive were of the myeloid/macrophage lineage (CD163+), not prostate tumor cells (PSMA+), as shown in Figure 3D. Increased IDO staining in tumors was generally associated with higher serum kyn:trp ratios (Figure S4, n=8, p=0.12).
Figure 3: Patients with prostate cancer treated with PD-1 blockade and/or an anti-tumor vaccine develop increased IDO expression in prostate tumor microenvironment.
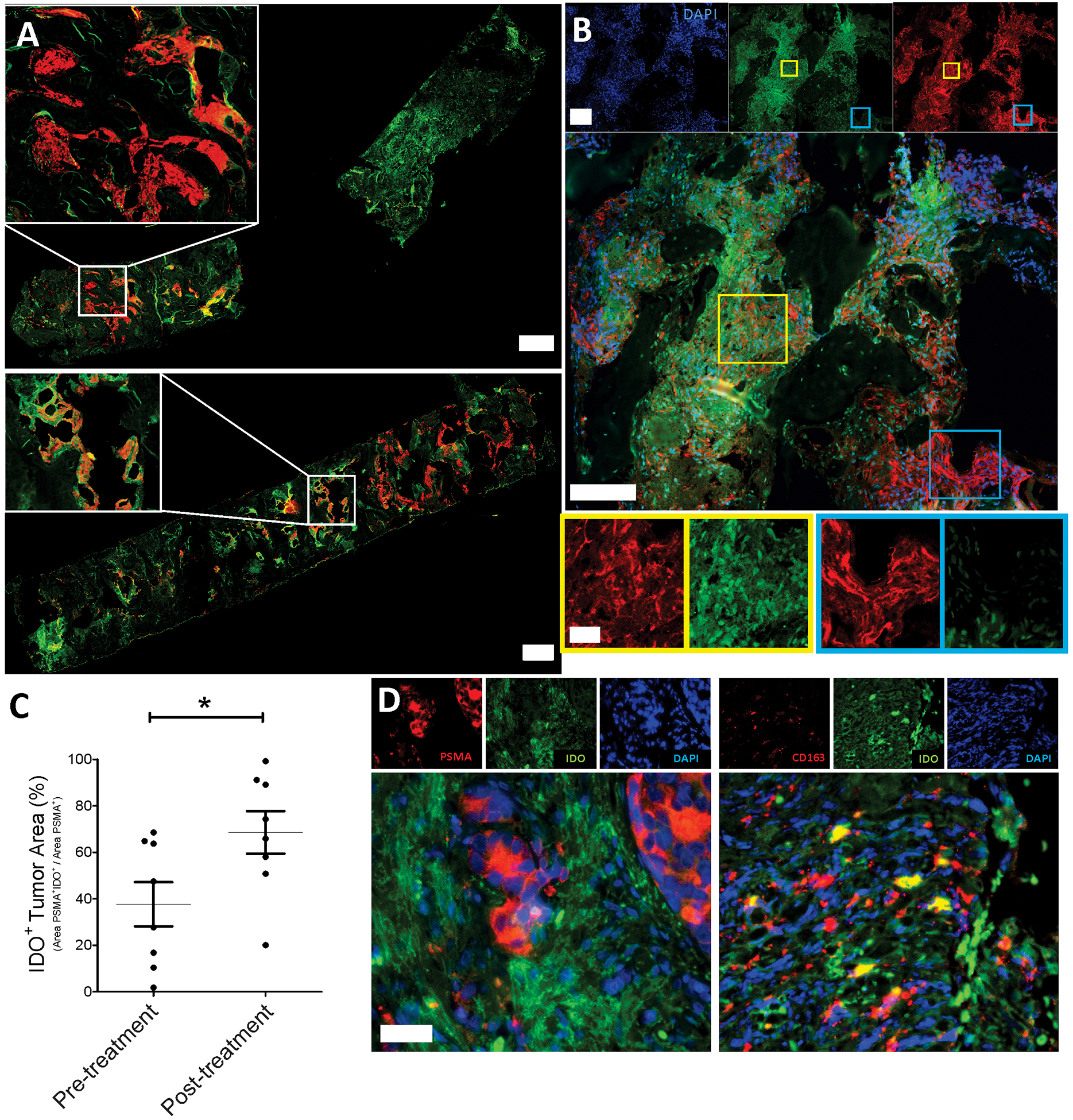
Metastatic tissue biopsies obtained pre-treatment and at 12 weeks were obtained from 8 patients and evaluated by immunofluorescence for DAPI, IDO, and PSMA expression. Panel A: Shown are entire sections from one individual at baseline (pre) and week 12 (post) demonstrating variable expression in different tumor sections (10x magnification, scale bars = 1000μm). Further examples are shown in Figure S2. Panel B: Higher magnification (40x, scale bars = 200μm top four images, scale bar = 50μm bottom four images) to demonstrate individual and composite staining with immunofluorescent staining for PSMA (red)/IDO (green)/DAPI (blue). Panel C: Quantification of IDO staining within PSMA+ tumor regions for all samples. Panel D: Immunofluorescent staining of a representative post-treatment tumor sample for PSMA (red)/IDO (green)/DAPI (blue, left) and CD163 (red)/IDO (green)/DAPI (blue, right) (20x magnification, scale bar = 100 μm) Statistical comparison was made using a paired Wilcoxon signed rank test.
IDO activity is associated with modest decrease in vaccine antigen-specific T-cell function.
The observation that increased kyn:trp ratios were associated with absence of PSA declines suggested that increased IDO expression might be a mechanism of tumor resistance to antigen-specific T cells elicited with vaccination. To test this, peripheral blood cells obtained from 22 patients after treatment with vaccine and pembrolizumab were evaluated for PAP antigen-specific IFNγ release in the presence or absence of 1-methyltryptophan, an IDO inhibitor that has been demonstrated to enhance T-cell activation in vitro (22). As shown in Figure 4, while not statistically different with this small sample size, an increase in the detection of IFNγ-secreting PAP-specific cells could be observed in the presence of 1-methyltryptophan.
Figure 4: IDO activity is associated with decreased vaccine antigen-specific T-cell function.
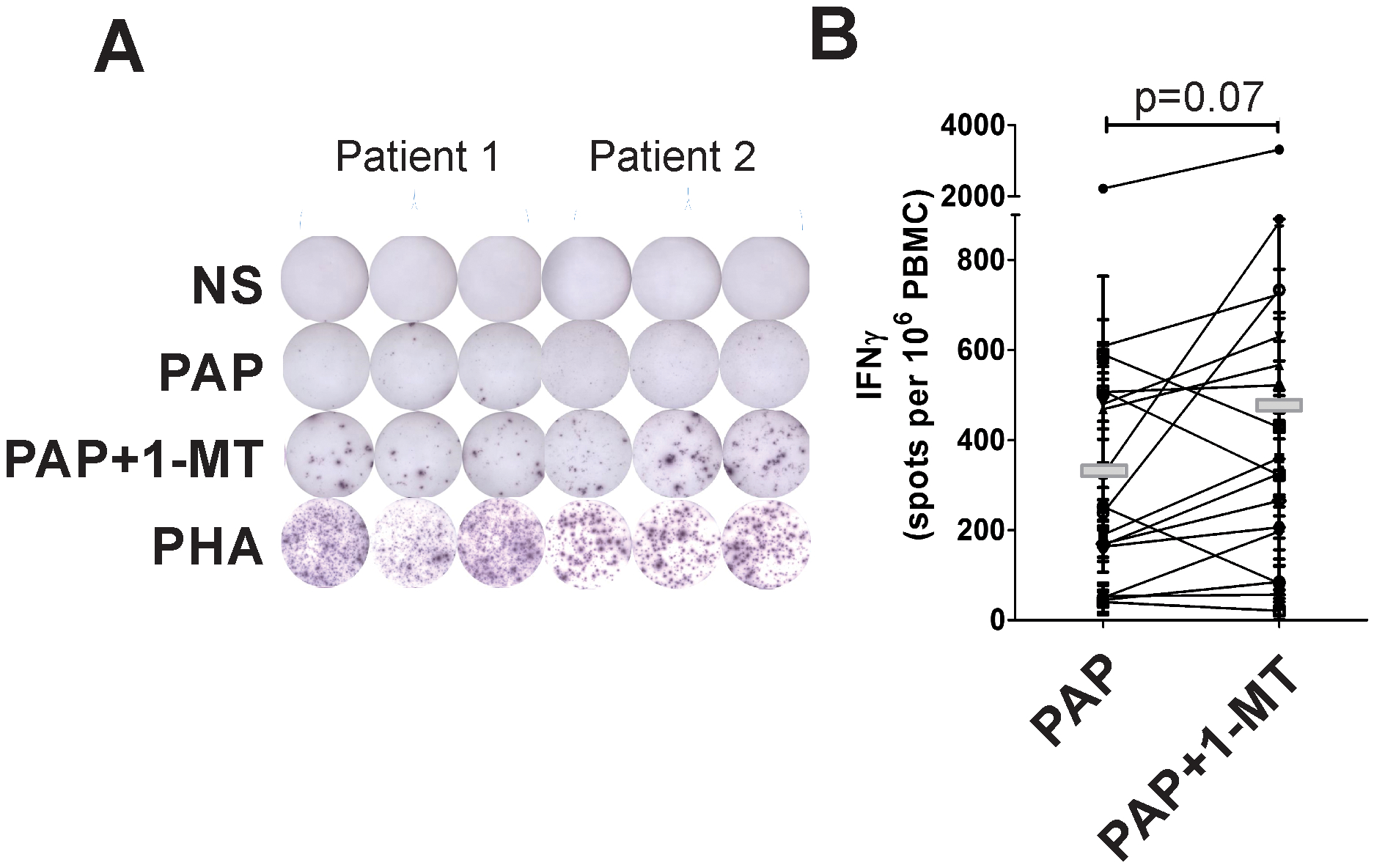
Peripheral blood mononuclear cells (PBMC) were obtained from patients treated with vaccine with or without pembrolizumab (after 12 weeks of treatment). PBMC were cultured in the presence of an overlapping peptide library derived from the PAP vaccine antigen (PAP), media alone, or phytohemaglutinin (PHA) as a positive control. Cells were also cultured with antigen in the presence of 1-MT. IFNγ-secreting T cells were detected by ELISPOT. Shown are representative ELISPOTS from two subjects (panel A), and cumulative data from 22 subjects evaluated (panel B). Statistical comparisons were made with a two-sided paired t test.
DISCUSSION:
In this report, we found that kyn:trp ratios were increased in patients with advanced prostate cancer. Because prostate cancer is a disease associated with more advanced age, we evaluated kyn:trp ratios with respect to age. Kyn:trp ratios were highly associated with patient age, and less associated with overall tumor burden, at least as measured by serum PSA. However, increased IDO activity did not appear to be independent of prostate cancer, because kyn:trp ratios were markedly induced, and to higher levels, following only 12 weeks of immunotherapy treatment with either a tumor vaccine or pembrolizumab, an effect independent of patient age or tumor volume. Our findings are consistent with previous reports demonstrating increased IDO gene expression in human prostate tumors relative to benign prostate tissue (10). Studies in TRAMP mice similarly showed expression of IDO in prostate tumors, and found that genetic crosses leading to the disruption of IDO activity delayed the development of prostate tumors (9). Together, these results, with our immunohistochemistry findings, suggest that IDO is expressed in the prostate tumor microenvironment, and that expression may be associated with disease progression, but that the expression may be most influenced by the presence of a T-cell immune response to the tumor. Of note, different cell types, including myeloid cells, can express IDO. Our studies suggest that the majority of the IDO expression within prostate tumors comes from CD163+ cells, notably cells of myeloid lineage including myeloid-derived suppressor cells (MDSC) and M2 macrophages. Notwithstanding, it is conceivable that IDO is produced by other cells, including tumor cells, and is taken up by these phagocytic cells.
IDO expression by human cells is known to be induced by IFNγ (18). In a recent report by Banzola and colleagues, the investigators evaluated prostate tumor cell lines and primary prostate cancer cells. They observed that IDO gene expression was higher in prostate cancer cells compared to benign tissues, associated with the expression of IFNγ and its receptors, and inducible in prostate cancer cell lines following IFNγ stimulation. Moreover, higher expression was associated with higher risk of biochemical recurrence following primary treatment (12). Our results demonstrate that immunotherapy treatment of prostate cancer, by either anti-tumor vaccine or PD-1 blockade, can increase IDO expression and activity. These effects are likely mediated by IFNγ released by lymphocytes activated by these treatments, consistent with our findings that serum IFNγ concentrations were highly associated with IDO activity, although this could not be definitively assessed. These findings are also consistent with our previous studies demonstrating that PD-L1, another protein induced following IFNγ exposure, is similarly expressed at higher levels following therapeutic anti-tumor vaccination in both murine models and in patients following treatment with this same PAP DNA vaccine (14, 15).
The finding that IDO expression is specifically increased following treatments that activate tumor-specific CD8+ T cells, notably by either vaccination or PD-1 blockade, suggests that it is a mechanism of immune evasion used by prostate cancer. This is further suggested by our finding that IDO expression was most induced in patients who did not experience evidence of anti-tumor response, as measured by any PSA decline, with immunotherapy treatment, whereas IDO activity was stable or decreased in patients with evidence of PSA decline. Curiously, some patients treated with combination therapy, previously demonstrated to elicit CD8+ T cell infiltration into tumors, did not have detectable increases in serum IDO activity. We suspect this was due to decreased tumor volume that was observed in these individuals treated with the combination (17). However, it is also possible that this was due to patient-specific pretreatment difference in their ability to mount IDO expression or due to differences in tumor volume or myeloid cell infiltrates, either of which could affect IDO activity and tumor response. Finally, we demonstrated that the tumor-specific T cells elicited with antigen-specific vaccination had decreased activity associated with IDO, as measured by antigen-specific IFNγ secretion by T cells, as this could be reversed in the presence of 1-methyltryptophan. These findings suggest that T-cell directed immunotherapy might be improved in the presence of IDO inhibition, or treatments aimed at reducing myeloid cells that may be producing IDO. Other preclinical studies have demonstrated that IDO activity is a mechanism of resistance to T-cell checkpoint blockade (7, 23). However, a recent clinical trial in patients with melanoma did not show any benefit to using an IDO inhibitor with PD-1 blockade (24). The use of these agents with anti-tumor vaccination, an approach that can lead to increased number of T cells secreting IFNγ, however, should be explored. Of note, one phase II trial has evaluated 1-methyltryptophan (indoximod) following treatment with the prostate cancer vaccine sipuleucel-T, however final results from that trial are pending (25). Future studies will explore this approach directly in animal models and other human clinical vaccine trials.
CONCLUSIONS:
In this report we found that IDO activity is increased in patients with more advanced prostate cancer and this activity is augmented following prostate tumor-directed immunotherapy. This was detected both systemically, by evaluating kynurenine and tryptophan concentrations in the peripheral blood, and also evaluating for IDO expression in prostate tumor biopsies. The observations that expression was increased primarily in patients who did not have evidence of anti-tumor effect, and that IDO inhibition increased the effector function of vaccine-induced T cells, suggests that it is a specific mechanism of immune resistance in prostate cancer. Together, these findings suggest that IDO inhibition should be explored in combination with T-cell activating immunotherapies targeting prostate cancer.
Supplementary Material
Figure S1: Kynurenine concentration are higher in sera of patients with advanced prostate cancer. Sera or plasma samples were evaluated for kynurenine concentrations from normal male volunteer blood donors (n=12), patients with newly diagnosed prostate cancer pre-treatment (n=14), patients with non-castrate, PSA-recurrent non-metastatic (M0) prostate cancer (n=15), castration-resistant, M0 prostate cancer (n=15), and castration-resistant, metastatic prostate cancer (n=16). Shown are the concentrations of kynurenine for each group (panel A), and overall with respect to subject age (panel B) or serum PSA for individuals with prostate cancer (panel C). Open circles in panel B are normal male blood donors. For panel A, * = p< 0.05 (Kruskal-Wallis test with Dunn’s correction for multiple comparisons). Tests of correlation with age and PSA (panels B and C) were made by Spearman test.
Figure S2: IDO, PSMA, and DAPI staining of metastatic prostate cancer tissues pre- and post-treatment. Shown are examples of IF staining from three additional patients separate from that shown in Figure 3. Top panels in each are from biopsies obtained pre-treatment, and bottom panels are from biopsies from the same metastatic site biopsied after immunotherapy (vaccine +/− anti-PD1) treatment. For each panel stains are DAPI (blue), PSMA (red), and IDO (green). Bars in the corners indicate size = 1000 μm.
Figure S3: Methods for quantification of IDO staining within tumor regions. Whole FFPE tumor biopsies were stained with IDO, PSMA, and DAPI as described in the Methods section. Whole section mosaic images were obtained on the Leica DMi8 at 10x (Panels A-B). Using the image in panel B, steps 1–7 depict the image processing steps taken to quantify the area of IDO expression as a percent of area of PSMA. First, a threshold was determined for the original greyscale images using the ImageJ built-in IsoData algorithm for IDO and Huang algorithm for PSMA (Steps 1–2). The threshold was applied, and the image converted into a binary mask. Because PSMA is a membrane stain, for that protein the conversion was followed by the “fills holes” ImageJ function (Steps 3–3b). Once the binary images were created a selection was made using the ImageJ built-in function (edit/selection/create selection) and the area quantified using the measure function (process/measure) to give total tumor (AT) and IDO-expressing (AI) areas (Step 4). To calculate the percentage of AT that overlapped with AI, the selection of IDO was applied to the mask of PSMA, filled white, and the remaining tissue area measured as described above. The process was repeated for PSMA over IDO (Steps 5–7).
Figure S4: Association of tumor expression of IDO and serum kyn:trp ratio. Quantitative imaging performed as in Figure 3 was used to determine the % IDO staining within tumor regions. These values are shown in relation to serum kyn:trp ratios obtained from the same individuals at the same time points (n=8). Test of correlation was made by Spearman test.
Acknowledgments:
We are grateful to Dr. Robert Newton (Incyte) and Worldwide Clinical Trials for conducting analysis of tryptophan and kynurenine concentrations in blood samples, and to Dr. Glenn Liu for helpful comments on the manuscript.
Funding: This work was supported by the Prostate Cancer Foundation (2014 Movember-PCF Challenge Award) and by National Institutes of Health R01 CA219154 and P30 CA014520.
LIST OF ABBREVIATION:
- 1-MT
1-methyl-tryptophan
- CTLA-4
Cytolytic T lymphocyte antigen 4
- DNA
Deoxyribonucleic acid
- ELISA
Enzyme-linked immunosorbent assay
- ELISPOT
Enzyme-linked immunosorbent spot assay
- IDO
Indoleamine 2,3-dioxygenase
- IF
Immunofluorescent
- IFNγ
Interferon-gamma
- IHC
Immunohistochemistry
- IRB
Institutional review board
- kyn
Kynurenine
- LC/MS
Liquid chromatography / mass spectrometry
- mCRPC
Metastatic, castration-resistant prostate cancer
- PAP
Prostatic acid phosphatase
- PBMC
Peripheral blood mononuclear cells
- PD-(L)1
Programmed death-1 (ligand)
- PSA
Prostate-specific antigen
- TIL
Tumor-infiltrating lymphocyte
- TRAMP
Transgenic adenocarcinoma of mouse prostate
- trp
Tryptophan
Footnotes
Conflicts of Interest: Douglas G. McNeel has ownership interest, has received research support, and serves as consultant to Madison Vaccines, Inc. which has licensed intellectual property related to this content. None of the other authors have relevant potential conflicts of interest.
Ethical approval and ethical standards: Samples were collected under University of Wisconsin IRB-approved protocols, and all patients gave written, informed consent for remaining samples to be used for research. Long-term use of blood samples collected from research subjects who had previously consented for remaining samples to be used for immunology-related research was approved on 11/21/2016 under University of Wisconsin IRB protocol 2013–0126-CR004 as a minimal risk protocol, not requiring additional patient consent.
REFERENCES:
- 1.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell. 144: 646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature medicine. 9: 1269–74. doi: 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 3.Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, Taylor R, Brew BJ (2011) Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PloS one. 6: e14698. doi: 10.1371/journal.pone.0014698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH (2015) The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines. 3: 703–29. doi: 10.3390/vaccines3030703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munn DH, Mellor AL (2016) IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends in immunology. 37: 193–207. doi: 10.1016/j.it.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD (2015) Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell reports. 13: 412–24. doi: 10.1016/j.celrep.2015.08.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP (2013) Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 210: 1389–402. doi: 10.1084/jem.20130066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komiya T, Huang CH (2018) Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Frontiers in oncology. 8: 423. doi: 10.3389/fonc.2018.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallberg E, Wikstrom P, Bergh A, Ivars F, Leanderson T (2010) Indoleamine 2,3-dioxygenase (IDO) activity influence tumor growth in the TRAMP prostate cancer model. Prostate. 70: 1461–70. doi: 10.1002/pros.21181 [DOI] [PubMed] [Google Scholar]
- 10.Feder-Mengus C, Wyler S, Hudolin T et al. (2008) High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur J Cancer. 44: 2266–75. doi: 10.1016/j.ejca.2008.05.023 [DOI] [PubMed] [Google Scholar]
- 11.Kolijn K, Verhoef EI, Smid M, Bottcher R, Jenster GW, Debets R, van Leenders G (2018) Epithelial-Mesenchymal Transition in Human Prostate Cancer Demonstrates Enhanced Immune Evasion Marked by IDO1 Expression. Cancer Res. 78: 4671–9. doi: 10.1158/0008-5472.CAN-17-3752 [DOI] [PubMed] [Google Scholar]
- 12.Banzola I, Mengus C, Wyler S et al. (2018) Expression of Indoleamine 2,3-Dioxygenase Induced by IFN-gamma and TNF-alpha as Potential Biomarker of Prostate Cancer Progression. Frontiers in immunology. 9: 1051. doi: 10.3389/fimmu.2018.01051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Blanco G, Burgers PC, Dekker LJ, Vredenbregt-van den Berg MS, Ijzermans JN, Schenk-Braat EA, Jenster G, Luider TM (2014) Serum kynurenine/tryptophan ratio is not a potential marker for detecting prostate cancer. Clinical biochemistry. 47: 1347–8. doi: 10.1016/j.clinbiochem.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 14.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG (2015) PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer immunology research. 3: 946–55. doi: 10.1158/2326-6066.CIR-14-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rekoske BT, Olson BM, McNeel DG (2016) Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. Oncoimmunology. 5: e1165377. doi: 10.1080/2162402X.2016.1165377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahm CD, Colluru VT, McNeel DG (2017) Vaccination with High-Affinity Epitopes Impairs Antitumor Efficacy by Increasing PD-1 Expression on CD8+ T Cells. Cancer immunology research. 5: 630–41. doi: 10.1158/2326-6066.CIR-16-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, Liu G (2018) Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 9: 25586–96. doi: 10.18632/oncotarget.25387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H (1991) Induction of indoleamine 2,3-dioxygenase in human cells in vitro. Advances in experimental medicine and biology. 294: 505–9. [DOI] [PubMed] [Google Scholar]
- 19.Colluru VT, Zahm CD, McNeel DG (2016) Mini-intronic plasmid vaccination elicits tolerant LAG3+ CD8+ T cells and inferior antitumor responses. Oncoimmunology. 5: e1223002. doi: 10.1080/2162402x.2016.1223002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindelin J, Arganda-Carreras I, Frise E et al. (2012) Fiji: an open-source platform for biological-image analysis. Nature methods. 9: 676–82. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeel DG, Becker JT, Eickhoff JC et al. (2014) Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res. 20: 3692–704. doi: 10.1158/1078-0432.ccr-14-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH (2007) Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 67: 792–801. doi: 10.1158/0008-5472.CAN-06-2925 [DOI] [PubMed] [Google Scholar]
- 23.Brown ZJ, Yu SJ, Heinrich B et al. (2018) Indoleamine 2,3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol Immunother. doi: 10.1007/s00262-018-2190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long GV, Dummer R, Hamid O et al. (2018) Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: Results of the phase 3 ECHO-301/KEYNOTE-252 study. 2018 ASCO Annual Meeting. Abstract #108. [Google Scholar]
- 25.Jha GG, Gupta S, Tagawa ST, Koopmeiners JS, Vivek S, Dukdek AZ, Cooley SA, Blazar BR, Miller JS (2017) A phase II randomized, double-blind study of sipuleucel-T followed by IDO pathway inhibitor, indoximod, or placebo in the treatment of patients with metastatic castration resistant prostate cancer (mCRPC). 2017 ASCO Annual Meeting. pp. Abstract #3066 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Kynurenine concentration are higher in sera of patients with advanced prostate cancer. Sera or plasma samples were evaluated for kynurenine concentrations from normal male volunteer blood donors (n=12), patients with newly diagnosed prostate cancer pre-treatment (n=14), patients with non-castrate, PSA-recurrent non-metastatic (M0) prostate cancer (n=15), castration-resistant, M0 prostate cancer (n=15), and castration-resistant, metastatic prostate cancer (n=16). Shown are the concentrations of kynurenine for each group (panel A), and overall with respect to subject age (panel B) or serum PSA for individuals with prostate cancer (panel C). Open circles in panel B are normal male blood donors. For panel A, * = p< 0.05 (Kruskal-Wallis test with Dunn’s correction for multiple comparisons). Tests of correlation with age and PSA (panels B and C) were made by Spearman test.
Figure S2: IDO, PSMA, and DAPI staining of metastatic prostate cancer tissues pre- and post-treatment. Shown are examples of IF staining from three additional patients separate from that shown in Figure 3. Top panels in each are from biopsies obtained pre-treatment, and bottom panels are from biopsies from the same metastatic site biopsied after immunotherapy (vaccine +/− anti-PD1) treatment. For each panel stains are DAPI (blue), PSMA (red), and IDO (green). Bars in the corners indicate size = 1000 μm.
Figure S3: Methods for quantification of IDO staining within tumor regions. Whole FFPE tumor biopsies were stained with IDO, PSMA, and DAPI as described in the Methods section. Whole section mosaic images were obtained on the Leica DMi8 at 10x (Panels A-B). Using the image in panel B, steps 1–7 depict the image processing steps taken to quantify the area of IDO expression as a percent of area of PSMA. First, a threshold was determined for the original greyscale images using the ImageJ built-in IsoData algorithm for IDO and Huang algorithm for PSMA (Steps 1–2). The threshold was applied, and the image converted into a binary mask. Because PSMA is a membrane stain, for that protein the conversion was followed by the “fills holes” ImageJ function (Steps 3–3b). Once the binary images were created a selection was made using the ImageJ built-in function (edit/selection/create selection) and the area quantified using the measure function (process/measure) to give total tumor (AT) and IDO-expressing (AI) areas (Step 4). To calculate the percentage of AT that overlapped with AI, the selection of IDO was applied to the mask of PSMA, filled white, and the remaining tissue area measured as described above. The process was repeated for PSMA over IDO (Steps 5–7).
Figure S4: Association of tumor expression of IDO and serum kyn:trp ratio. Quantitative imaging performed as in Figure 3 was used to determine the % IDO staining within tumor regions. These values are shown in relation to serum kyn:trp ratios obtained from the same individuals at the same time points (n=8). Test of correlation was made by Spearman test.


