Fig. 3 |. Interplay between epigenetics and metabolism.
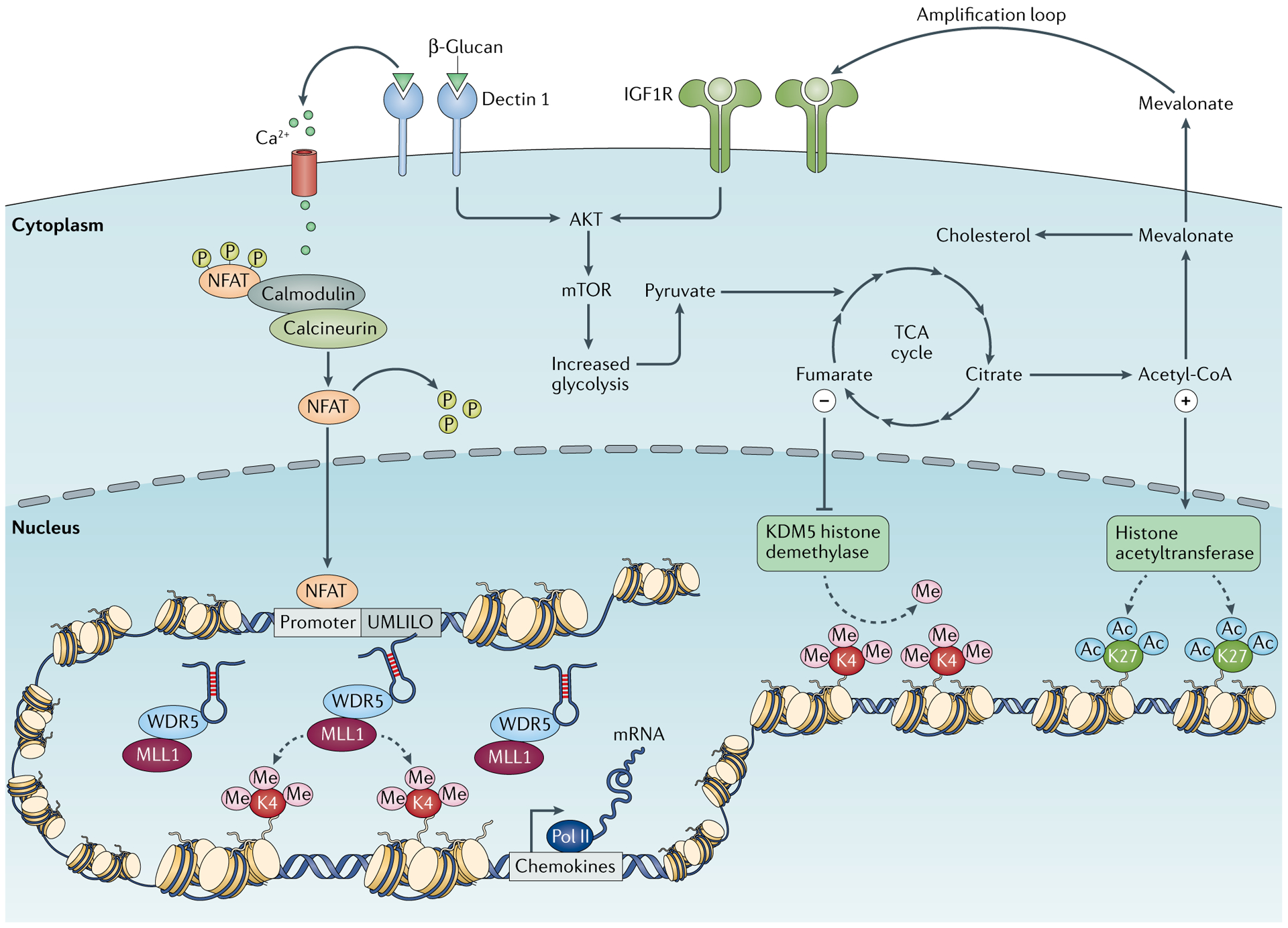
The correct initiation of the mechanisms necessary for the induction of trained immunity relies on the active interplay between epigenetic and metabolic reprogramming of the innate immune cells on stimulation. During primary challenge, the recognition of specific ligands by pattern recognition receptors triggers a series of intracellular cascades that lead to the upregulation of different metabolic pathways, such as glycolysis, tricarboxylic acid (TCA) cycle and fatty acid metabolism. Certain metabolites derived from these processes, such as fumarate and acetyl coenzyme A (acetyl-CoA), can activate or inhibit a series of enzymes involved in remodelling the epigenetic landscape of cells, such as the histone demethylase lysine-specific demethylase 5 (KDM5) or histone acetyltransferases, leading to specific changes in histone methylation and acetylation of genes involved in the innate immune responses. β-Glucan-mediated activation of dectin 1 signalling also triggers calcium influx, which leads to the dephosphorylation of nuclear factor of activated T cells (NFAT), allowing its translocation into the nucleus, where it may bind to DNA and activate gene transcription. This facilitates the accessibility of the DNA to the transcriptional machinery and gene regulatory elements and specific long non-coding RNAs, promoting and facilitating an enhanced gene transcription on secondary stimulation of the cells. IGF1R , insulin-like growth factor 1 receptor ; MLL1, mixed-lineage leukaemia protein 1 (also known as histone-lysine N-methyltransferase 2A); mTOR , mechanistic target of rapamycin; Pol, polymerase; UMLILO, upstream master long non-coding RNA of the inflammatory chemokine locus; WDR5, WD repeat-containing protein 5.
