Fig. 3.
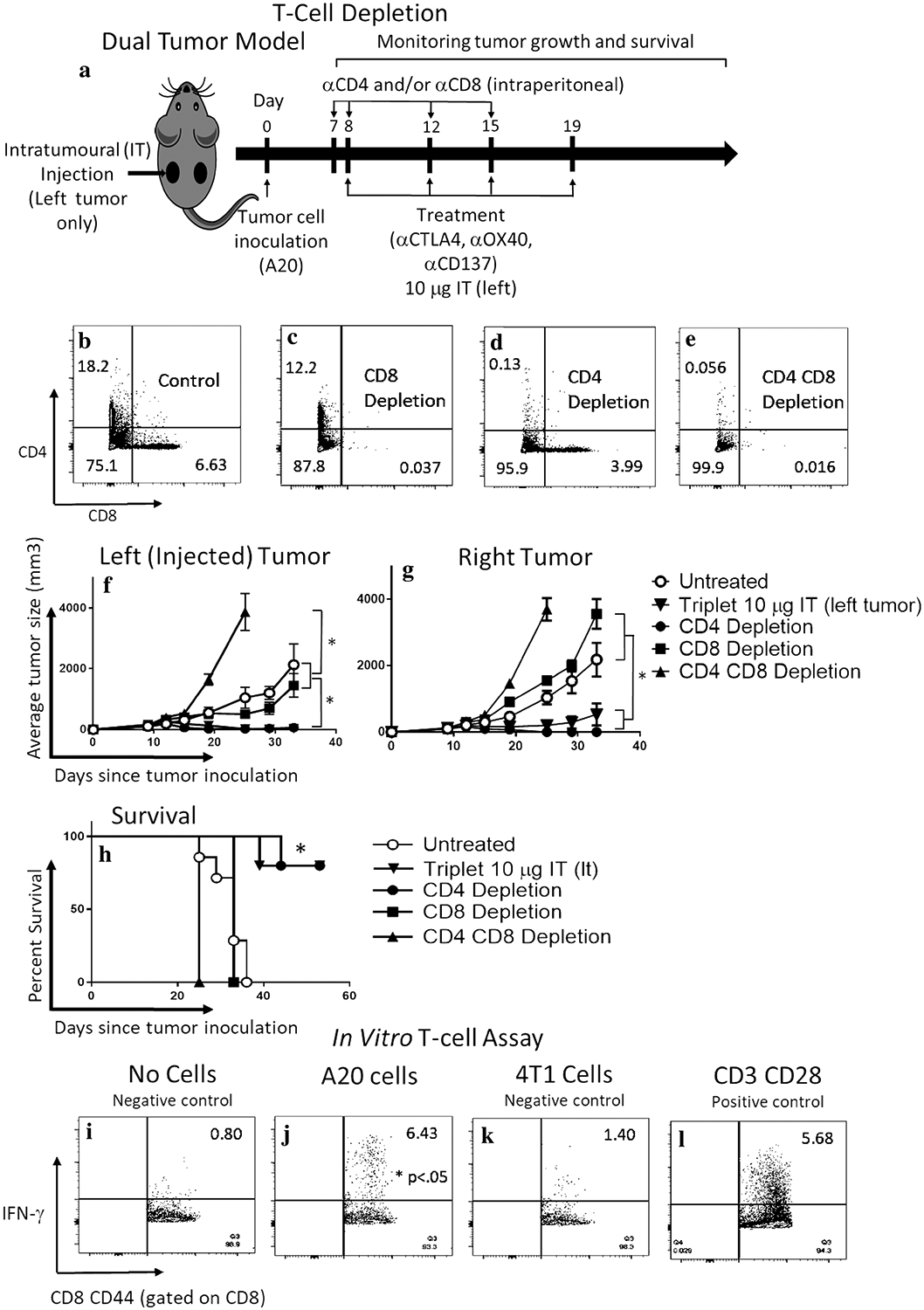
CD8 T-cell is required for efficacy, and CD8CD44hi memory cells generate a specific anti-tumor response ex vivo. a In vivo T-cell depletion. Mice were treated with four biweekly doses of the triple combination αCD137, αOX40, and αCTLA4 at the 10 μg dose delivered intratumorally to the left tumor of a dual tumor A20 lymphoma model. CD8 and/or CD4 cells were depleted by systemic (intraperitoneal) administration of respective antibodies. Dot-plots panels showing peripheral blood staining of CD4 and CD8 T cells in non-depleted (b), CD8 (c), CD4 (d), or CD4 and CD8 (e) depleted mice. Tumor measurement by caliper of injected (f) or non-injected (g) mice after no depletion, CD4, CD8, CD4 and CD8 depleted and non-depleted mice treated with the triple combination. h Survival curve. ‘*’ denotes p < .05 (Student’s t test for tumor growth and Mantel-Cox for survival). Values are expressed as mean ± SEM. n = 5 per group. Splenocytes from mice treated with the triplet combination that had cleared their tumors were incubated overnight with i CD28 alone (negative control), j CD28 + A20 cells, k CD28 + 4T1 breast cancer cells—an irrelevant syngeneic cancer cell line (negative control), or l CD28 + CD3 (positive control). IFN-γ production by CD8CD44 T cells was evaluated by flow cytometry. Numbers in upper right corner represent percentage of positive cells. ‘*’ denotes p < .05 (Student’s t test), n = 3 per group
