Figure 4. Deletion of Lpar1 in neuronal lineages protects against PSNL induced neuropathic pain.
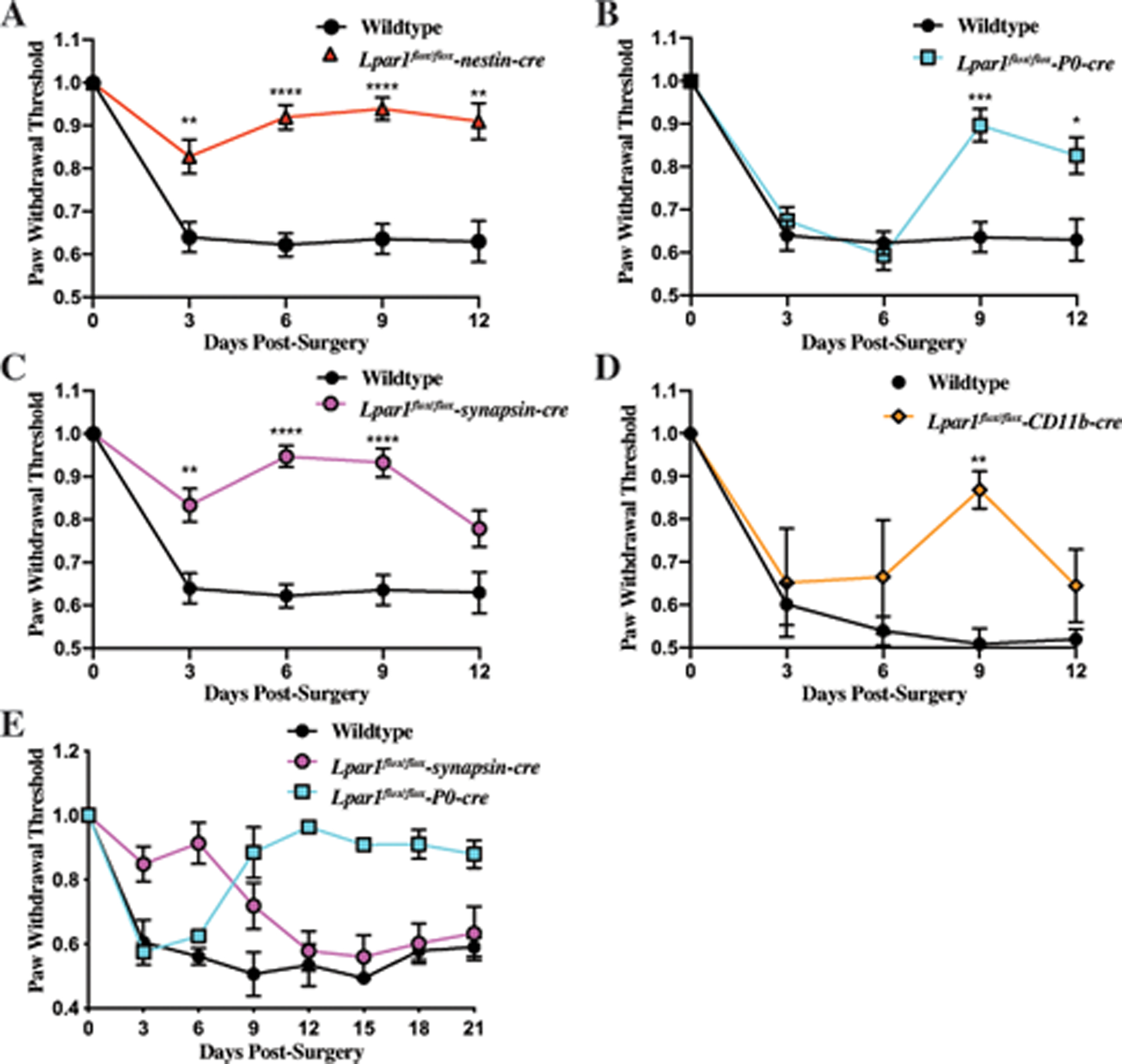
(A) Targeted nestin-cre-mediated deletion of Lpar1 in all neural lineages protects against neuropathic pain in the PSNL mouse model. (B) Schwann cell specific deletion of Lpar1 through a P0-cre transgene protects mice from PSNL at later but not earlier time points. (C) Specific deletion of Lpar1 in neurons protects mice from PSNL induced neuropathic pain at early time points but not at later time points. (D) Deletion of Lpar1 in CD11b expressing cell types provides protection from neuropathic pain at day 9 post-PSNL. (E) Schwann cell specific deletion of Lpar1 occurs at later time points and is long-lasting. The plotted data is the average paw withdrawal threshold time observed for Lpar1 conditional null mutants normalized to Lpar1flox/flox control animal responses +/− SEM. For (A, B, and C), N=10 Lpar1flox/flox, N=10 Lpar1flox/flox-nestin-cre, N=9 Lpar1flox/flox-P0 cre, and N=8 Lpar1flox/flox-synapsin-cre animals. For (D), N=4 Lpar1flox/flox, and N=4 Lpar1flox/flox-CD11b-cre. For (E), N=2 for all genotypes used. Statistical analysis was performed using a two-way Anova, followed by a Sidak’s multiple comparisons test, differences were considered significant when P≤0.05 (*=P≤0.05, **≤.001, ***≤0.001, ****P≤0.0001).
