Abstract
Purpose:
To analyze a case series of retinal vasculitis reported to the American Society of Retina Specialists (ASRS) following Food and Drug Administration approval of brolucizumab for treatment of neovascular age-related macular degeneration.
Methods:
The ASRS Research and Safety in Therapeutics Committee analyzed clinical and imaging characteristics from submitted reports of retinal vasculitis after brolucizumab.
Results:
Retinal vasculitis was reported in 26 eyes of 25 patients (22 [88%] female) after treatment with brolucizumab. Imaging studies were available for 24 of 26 eyes. Most cases (92%) were associated with intraocular inflammation, which presented at a mean of 25 days (range, 3–63 days) after the most recent brolucizumab injection. Mean visual acuity (VA) was 20/52 (range, 20/25–4/200) before the adverse event, 20/151 (range, 20/25-hand motion) at presentation of the adverse event, and 20/243 (range, 20/30-light perception) at last follow-up. Twelve eyes (46%) had a greater than 3-line decrease in VA at final follow-up, and 12 eyes (46%) had a final VA of 20/200 or worse. Analysis of retinal imaging identified vasculopathy that involved retinal arteries (91%), retinal veins (79%), and choroidal vessels (48%). Occlusive disease was apparent on imaging in 83% of eyes. Treatment approaches were varied.
Conclusions:
Retinal vasculitis has been identified in a series of eyes following brolucizumab. Although a few eyes in this series were asymptomatic or minimally symptomatic, some eyes had significant vision loss. A careful examination for signs of active inflammation prior to brolucizumab injection is recommended. Once vasculopathy is suspected, angiographic imaging may help define the spectrum of involvement. Optimal treatment strategies remain unknown.
Keywords: anti-VEGF agents, branch retinal artery occlusion, central retinal artery occlusion, uveitis, vitritis
Introduction
On October 7, 2019, brolucizumab 6 mg (Beovu, Novartis International AG) was approved by the US Food and Drug Administration (FDA) for treatment of neovascular age-related macular degeneration (NVAMD), with the hope of reducing treatment burden compared with the other antivascular endothelial growth factor (anti-VEGF) agents. Brolucizumab is a single-chain antibody fragment that blocks all forms of VEGF-A and can be concentrated to give higher doses because of its highly soluble nature and small molecular weight. The phase 3 HAWK and HARRIER studies demonstrated that brolucizumab had a greater drying effect on the retina than aflibercept. Approximately 50% of patients could be maintained on an every-12-week brolucizumab dosage with noninferior visual acuity (VA) outcomes compared with an every-8-week aflibercept regimen.1
Shortly following the FDA approval of brolucizumab, the American Society of Retina Specialists (ASRS) began receiving reports of inflammation following intravitreal brolucizumab administration for NVAMD. In addition to cases of intraocular inflammation, several reported cases included retinal vasculitis that frequently resulted in vascular occlusion and significant vision loss. Two case reports were recently published describing this phenomenon.2,3 The purpose of this study is to analyze the characteristics of postapproval cases of retinal vasculitis voluntarily reported to the ASRS as of April 1, 2020.
Methods
The ASRS Research and Safety in Therapeutics (ReST) Committee collected and analyzed clinical and imaging data from submitted reports of retinal vasculitis that occurred after intravitreal brolucizumab and were reported to the ASRS as of April 1, 2020. Cases that were deemed most likely related to infectious endophthalmitis were excluded from the study. Data were tabulated with Microsoft Excel. Snellen VA was converted to logarithm of the minimum angle of resolution equivalents for the purpose of analysis. All patient information was deidentified.
Initial reports were submitted by treating physicians to the ASRS ReST Committee through the ASRS website (https://www.asrs.org/forms/4/asrs-adverse-event-report-form). The ReST Committee followed up with reporting physicians to collect a standardized data set including location; sex; age; eye; race; medical history (including autoimmune disease); allergy history; ocular history; date of NVAMD diagnosis; previous number and type of prior anti-VEGF therapy (including most recent therapy preceding brolucizumab); prior history of anti-VEGF–associated inflammation; reason for switching to brolucizumab; number and dates of brolucizumab injection(s); lot number of the causative brolucizumab injection; presence or absence of inflammation at time of brolucizumab injection; dates of presentation with an adverse event (AE) and all dates of subsequent follow-up; symptoms at AE presentation; presence or absence and location of intraocular inflammation, vasculitis, and vascular occlusion; treatment modalities; final follow-up date; presence or absence of inflammation at final follow-up (and time to resolution if applicable); residual examination findings; residual symptoms; date and type of anti-VEGF reinjection if available (including dates and whether there was recurrent inflammation); and anti-VEGF plan moving forward. VA and intraocular pressure from each visit were recorded.
Images including color photographs, fluorescein angiograms (FA), indocyanine green angiograms (ICGA), and optical coherence tomography (OCT) were collected when available. The images were then graded for presence or absence of findings independently by 2 graders (A.J.W. and P.H.); disagreements were resolved by open adjudication. If images were of insufficient quality to assess a given finding, they were deemed ungradable for that finding.
Color photographs were assessed for venous sheathing, venous beading, venous dilatation (in comparison to the fellow eye), venous tortuosity, arterial occlusion/sheathing/boxcarring, arteriolar narrowing (in comparison to the fellow eye), flame hemorrhages, dot/blot hemorrhages, macular whitening consistent with ischemia, extramacular whitening consistent with ischemia, and evidence of optic neuropathy (eg, swelling). FA images were assessed for arterial filling defects, venous filling defects, macular ischemia, extramacular ischemia, arterial staining/leakage, venous staining/leakage, optic nerve hyperfluorescence/leakage, and choroidal hypofluorescence. ICGA images were assessed for choroidal hypofluorescence. Choroidal hypofluorescence both on FA and ICGA was graded as zonal, multifocal, or both. Macular OCT images were assessed for inner retinal hyperreflectivity (consistent with acute ischemia), inner retinal thinning (consistent with ischemic sequelae), and paracentral acute middle maculopathy in absence of hyperreflectivity of other retinal layers.
Results
Demographics
Data from 26 eyes of 25 patients with reported retinal vasculitis after brolucizumab were included in the study. One additional reported case of retinal vasculitis after brolucizumab injection was deemed to be owing to infectious endophthalmitis and was excluded from the study. Patient demographics are summarized in Table 1. Twenty-two (88%) reported cases occurred in women. Mean age was 79 years, and 96% were white, consistent with the NVAMD population in the United States. Reported cases were seen throughout the United States (8 from the Northeast, 7 from Western states, 6 from Southern states, and 4 from the Midwest). There was no identifiable pattern for medical history, ocular history, or drug allergies. Five (20%) patients had a known history of inflammation (autoimmune disease or uveitis): 1 with multiple sclerosis, 1 with Raynaud syndrome and hypothyroidism, 1 with Graves disease, 1 with hypothyroidism and psoriasis, and 1 with a history of iritis.
Table 1.
Demographic Data.
| Characteristic | Value |
|---|---|
| No. of patients | 26 eyes of 25 patients (1 bilateral case) |
| Sex, No. (%) | |
| Female | 22 (88) |
| Male | 3(12) |
| Age, y, mean (range) | 79.1 (58–92) |
| Eye, No. of eyes (%) | |
| Right | 13 (50) |
| Left | 13 (50) |
| Race, No. (%) | |
| White | 24 (96) |
| Black | 1 (4) |
| Location, No. | |
| United States | 25 |
| Northeast | 8 |
| Western | 7 |
| Southern | 6 |
| Midwest | 4 |
| Autoimmune history, No. (%) | 5 (20): Multiple sclerosis Raynaud disease and hypothyroidism Graves disease Hypothyroidism and psoriasis History of iritis |
| Drug allergies, No. (%) | |
| No allergies | 14 (56%) |
| No pattern | 11 (44%) |
| Lens, No. of eyes (%) | |
| Phakic | 5(19) |
| Pseudophakic | 21 (81) |
| Months of NVAMD diagnosis prior to brolucizumab, No. (range) | 54.5 (4–138) |
| No. of prior anti-VEGF injections (before brolucizumab) | |
| Mean (range) | 39.1 (2–78) |
| Total | 1018 |
| Type of injections prior to brolucizumab, % | |
| Ranibizumab | 16 |
| Bevacizumab | 23 |
| Aflibercept | 61 |
| Most recent injection prior to brolucizumab, No. (%) | |
| Ranibizumab | 1 (4) |
| Bevacizumab | 6 (23) |
| Aflibercept | 19(73) |
| Days between last anti-VEGF and first brolucizumab, No. (range) | 43.4(19–111) |
| Reason to switch to brolucizumab, No. of eyes (%) | |
| Extend treatment interval Improve efficacy | 20 (77) |
| Worsening vision despite monthly | 19(73) |
| injections | 1 (4) |
Abbreviations: anti-VEGF, antivascular endothelial growth factor; NVAMD, neovascular age-related macular degeneration.
All patients had received previous treatment with other anti-VEGF agents with a mean number of 39 injections. The most recent anti-VEGF injection prior to brolucizumab was aflibercept in 19 eyes (73%), bevacizumab in 6 eyes (23%), and ranibizumab in 1 eye (4%). In all eyes, 1018 total prior anti-VEGF injections were given, of which 16% were ranibizumab, 23% were bevacizumab, and 61% were aflibercept. No eyes had a history of anti-VEGF–associated inflammation. Three patients had same-day bilateral injections of brolucizumab, and one patient developed retinal vasculitis in both eyes.
All AEs arose after 1 (11 eyes, 42%), 2 (11 eyes, 42%), or 3 (4 eyes, 16%) brolucizumab injections in the more than 5 months since approval (October 7, 2019) and 3 months since a permanent Healthcare Common Procedure Coding System (HCPCS) Level II alpha-numeric code (ie, “J-code”) had been established (January 1, 2020). The latest brolucizumab injection reported in this series was on February 17, 2020. There was no identifiable association with lot number (there were 8 different lot numbers provided from 20 injecting physicians). No brolucizumab injections were given in the presence of intraocular inflammation as noted by the reporting physician. Mean time to presentation was 26 days (range, 3–63 days) from the most recent brolucizumab injection, and 46 days (range, 15–146 days) from the first brolucizumab injection.
Symptoms and Examination Findings
Symptoms at AE onset included blurry vision (62%), floaters (46%), pain (31%), redness (19%), and scotomas (12%). Two (8%) eyes were asymptomatic and found to have only retinal vasculitis on routine follow-up examination. VA loss of 4 to 5 lines was noted at AE presentation (mean 20/151 from 20/52; see Table 2 for summary of VA data). Intraocular inflammation at AE presentation was identified in 24 (92%) eyes. The location of intraocular inflammation was anterior only in 8 (31%) eyes, posterior only in 7 (27%) eyes, both anterior and posterior in 9 (35%) eyes, and no intraocular inflammation was noted in 2 (8%) eyes (other than retinal vasculitis). In total, 17 (65%) eyes had anterior inflammation, with a mean grade of 1.4+ cells (range, 0.5–3+ cells), whereas 16 (62%) eyes had posterior inflammation (grade for vitritis was not collected). Mean intraocular pressure was 14.5 mm Hg (range, 9–28 mm Hg) at the time of most recent brolucizumab injection, and was 15.7 mm Hg (range, 9–28 mm Hg) at AE presentation.
Table 2.
Visual Acuity Data.
| Mean logMAR VA | Mean Snellen VA | Median Snellen VA | Range | |
|---|---|---|---|---|
| All eyes (n = 26) | ||||
| VA at first brolucizumab (baseline) | 0.3557 | 20/45 | 20/50 | 20/25 to 20/80 |
| VA at most recent brolucizumab | 0.4181 | 20/52 | 20/50 | 20/25 to 4/200 |
| VA at AE onset | 0.8781 | 20/151 | 20/70 | 20/25 to HM |
| Worst VA | 1.2977 | 20/397 | 20/150 | 20/30 to LP |
| VA at most recent follow-upa | 1.0861 | 20/243 | 20/80 | 20/30 to LP |
| Fellow eye VA | 0.4164 | 20/53 | 20/35 | 20/20 to HM |
| 12 eyes (46%) with < 20/200 VA and 12 eyes (46%) with > 3-line VA loss at last follow-up | ||||
| Eyes with > 60 days’ follow-up (n = 8) | ||||
| VA at first brolucizumab (baseline) | 0.4129 | 20/52 | 20/60 | 20/25 to 20/80 |
| VA at AE onset | 0.8605 | 20/145 | 20/70 | 20/40 to 2/200 |
| Worst VA | 1.4079 | 20/512 | 20/365 | 20/50 to LP |
| VA at most recent follow-upb | 1.0284 | 20/214 | 20/60 | 20/30 to LP |
| 3 eyes (38%) with ≤ 20/200 VA and 4 eyes (50%) with ≥ 3-line VA loss at last follow-up | ||||
Abbreviations: AE, adverse event; HM, hand motions; logMAR, logarithm of the minimum angle of resolution; LP, light perception; VA, visual acuity.
Mean 53 d since last brolucizumab.
Mean 86 d since last brolucizumab.
Among the 26 eyes with retinal vasculitis in this series, 22 (85%) eyes were reported by the treating physician as having occlusive vasculitis. In 16 eyes, occlusive vasculitis was seen at first AE presentation. One eye was initially reported to have nonocclusive vasculitis but subsequently developed occlusive vasculitis. The remaining 5 eyes first presented with intraocular inflammation only (no vasculitis) and then developed occlusive vasculitis at subsequent follow-up (mean 18 days between first AE presentation and appearance of vasculitis; range, 5–35 days).
Vasculitis Features
Images were available for 24 of 26 (92%) eyes. These included color photographs in 24 (92%) eyes, FA in 22 (85%) eyes, ICGA in 1 (4%) eye, and OCT in 18 (69%) eyes. A spectrum of vasculopathy was seen, ranging from minimal to severe. Vascular involvement included retinal arteries, retinal veins, and choroidal vessels. Optic nerve hyperfluorescence/leakage was noted on FA in 55% of cases. Retinal arteries were the most affected blood vessels (91% of eyes) (Table 3). Choroidal ischemia was noted in 10 of 21 eyes with angiography that was considered gradable for that finding. In the 1 eye that had both FA and ICGA imaging, multifocal areas of choroidal hypofluorescence were noted in late frames on ICGA but were not visible on FA.
Table 3.
Location of Pathology on Photos (n=24), Fluorescein Angiography (n=22), and Indocyanine Green Angiography (n=1).
| Arterial, 91% | Venous, 79% | Choroidal, 48% | Optic nerve, 55% |
|---|---|---|---|
Photos
|
Photos | Photos (choroid was not assessed using photos) | Photos |
|
|
||
| FA | FA | FA or ICGA: hypofluorescence (ischemia)a 48% | FA |
|
|
|
|
Abbreviations: FA, fluorescein angiography; ICGA, indocyanine green angiography.
Arterial only 21%, venous only 13%, arterial and venous together 67%.
Hypofluorescence observed in a deep multifocal and/or zonal pattern that persisted in late frames without leakage or staining was interpreted to be consistent with choroidal ischemia.
Occlusive vascular disease was defined by visualization of arterial occlusion, boxcarring, and/or sheathing on photographs, or presence of arterial and/or venous filling defects on FA. Retinal ischemia was defined as retinal whitening on photographs, nonperfusion on FA, and/or inner or middle retinal hyperreflectivity and thickening on OCT. Image analysis identified occlusive vascular disease in 20 (83%) eyes, consistent with 85% reported by the providers. In eyes with occlusive vascular disease on imaging, occlusive disease was arterial only in 9 (45%) eyes, venous only in none (0%), and both arterial and venous in 11 (55%) eyes. Image analysis showed evidence of ischemia in 21 (88%) eyes, of which the ischemia involved only the macula in 2 eyes (10%), only the peripheral retina in 3 eyes (14%), and both macula and peripheral retina in 16 (76%) eyes.
Outcomes
The most recent mean follow-up visit since AE onset was 23 days (median 17 days; range, 0–109 days), since the last brolucizumab injection was 53 days (median 59 days; range, 8–137 days), and since the first brolucizumab injection was 78 days (median 75 days; range, 21–140 days). At most recent follow-up, mean VA was 20/243 (median 20/80; range, 20/30-light perception). Twelve eyes (46%) had a more than 3-line decrease in VA at final follow-up, 9 eyes (35%) had a more than 6-line decrease in VA, and 12 eyes (46%) had a final VA of 20/200 or worse. A sensitivity analysis excluding eyes with less than 60 days’ follow-up after most recent brolucizumab injection revealed similar VA trends (see Table 2).
Residual symptoms at last reported follow-up included blurry vision in 16 (62%) eyes, floaters in 8 (31%) eyes, and scotomata in 2 (8%) eyes; 2 (8%) eyes had resolution of symptoms, and 2 (8%) eyes remained asymptomatic throughout. In terms of inflammation, 2 (8%) eyes had no intraocular inflammation throughout (other than vasculitis), 7 (27%) eyes had resolution of inflammation over a mean of 25 days (range, 7–64 days), and 17 (65%) eyes were reported to have inflammation at most recent follow-up. One patient, who developed occlusive retinal vasculitis 14 days after the second brolucizumab injection, developed a large subretinal hemorrhage 40 days after the second brolucizumab injection with subsequent hand motions vision. It was unclear whether this was related to NVAMD or secondary to vasculitis.
Treatment approaches were varied, and no trends were identifiable that could predict greater success with any specific approach. Twenty-four (92%) eyes were treated with topical corticosteroids, whereas 2 (8%) eyes received no treatment. Eleven (42%) patients received systemic corticosteroids, 5 (19%) eyes received intravitreal corticosteroid injections, and 4 (15%) eyes had a pars plana vitrectomy. One eye received intravitreal antibiotics, whereas 2 patients received antiviral medications.
Anti-VEGF Reinjection
Eight eyes were re-treated with a different anti-VEGF agent after diagnosis of an AE related to brolucizumab. Four of these eyes received aflibercept once inflammation had resolved, and there was no recurrence of inflammation in those eyes. In 2 eyes, there was persistent posterior inflammation reported at the time of retreatment with aflibercept, but there was no worsening of inflammation after aflibercept injection. In the last 2 eyes, onset of occlusive vasculitis and worsening of intraocular inflammation was noted to follow injection of anti-VEGF medication in the setting of persistent intraocular inflammation following brolucizumab injection given 1 month prior (one developed occlusive vasculitis 28 days after aflibercept and the other 21 days after ranibizumab).
Case Examples
Case 1.
A 72-year-old woman with NVAMD in the left eye and 30 prior anti-VEGF injections (most recently aflibercept) was switched to brolucizumab in attempt to extend treatment interval and improve anti-VEGF efficacy. She had a history of a benign meningioma, no other medical issues, and no known drug allergies. VA in the fellow eye was 20/30. She received 3 monthly brolucizumab injections in the left eye; VA was 20/50 at each visit before injection. Thirty-five days after her third brolucizumab injection, the patient was examined, and there was no inflammation or retinal vasculitis noted. Because there was no evidence of exudation on examination or OCT, the eye was observed until the next month without treatment. At the next visit, 63 days after the third brolucizumab injection, VA remained 20/50, and the patient stayed asymptomatic despite a recurrence of subretinal fluid on OCT. On examination, there was no intraocular inflammation reported, but occlusion within an inferotemporal arteriole was noted (Figure 1A). FA demonstrated arterial and venous filling defects in this area (Figure 1B). On this date, the eye was injected with aflibercept; the patient has not yet returned for her subsequent scheduled injection but has not reported any new symptoms.
Figure 1.

A 72-year-old woman with asymptomatic brolucizumab-associated occlusive retinal vasculopathy in the left eye, noted on routine follow-up 63 days after her third brolucizumab injection. (A) No intraocular inflammation was reported, but occlusion of an inferotemporal arteriole was visible. (B) Fluorescein angiography demonstrated arterial and venous filling defects with associated ischemia in this area, which persisted into the late phase and spared the macula.
Case 2.
A 92-year-old woman with NVAMD in the right eye and 65 prior anti-VEGF injections (most recently aflibercept) was switched to brolucizumab to extend treatment interval. She had a medical history of hypothyroidism and hypertension, ocular history of open-angle glaucoma and pseudophakia in both eyes, and allergy to briminodine. At the time of her first (and only) brolucizumab injection in the right eye, VA was 20/60. VA in the fellow eye was 20/70. Fourteen days later, she presented to the clinic with redness, pain, and blurry vision in the right eye. VA was 20/100, and examination revealed trace anterior chamber cells with inferior keratic precipitates. She was started on topical corticosteroids. Ten days later (24 days after brolucizumab), her pain had improved but her blurriness worsened. VA decreased to counting fingers at 4 feet, and posterior examination revealed vitritis, visible arterial occlusion/sheathing, and patchy retinal whitening consistent with retinal ischemia, with an inactive fibrotic scar in the central macula (Figure 2A). FA revealed retinal arterial and venous filling defects and hypoperfusion of the choroid in a large patch around the optic nerve as well as in several smaller multifocal areas; late-phase images demonstrated partial refilling of her choroidal defects and arteriolar leakage (Figure 2, B and C).
Figure 2.

A 92-year-old woman with intraocular inflammation in the right eye that subsequently progressed to symptomatic occlusive retinal vasculitis, noted 24 days after her first (and only) brolucizumab injection. (A) Posterior examination revealed vitritis, arterial sheathing/occlusion, and patchy retinal whitening most prominently in the peripapillary region. (B) Early-phase fluorescein angiography revealed retinal arterial and venous filling defects and choroidal hypofluorescence consistent with hypoperfusion in a large patch around the optic nerve as well as in several smaller multifocal areas. (C) Middle- and late-phase fluorescein angiography images demonstrated slowly resolving choroidal hypoperfusion, persistent arterial and venous filling defects, and focal leakage around arterioles superiorly and inferiorly.
Oral prednisone 60 mg was initiated. Ten days later, at the most recent follow-up (34 days after brolucizumab), prednisone was tapered to 40 mg. VA remained counting fingers at 4 feet. Examination findings were improving with no anterior chamber cells, mild vitritis, and improving but persistent retinal whitening.
Case 3.
A 78-year-old man with NVAMD in the left eye and 59 prior anti-VEGF injections (most recently bevacizumab) was switched to brolucizumab to extend treatment interval and improve anti-VEGF efficacy. He had a history of hypertension, no other ocular history, and an allergy to penicillin. VA in the fellow eye was 20/40.
At the time of his first (and only) brolucizumab injection in the left eye, VA was 20/60. Fourteen days after injection, the patient was seen at an outside clinic with redness and pain, and he was prescribed topical corticosteroids. Thirty-four days after brolucizumab, he presented to the retina clinic with redness, pain, blurry vision, and floaters. VA was hand motions, and examination revealed 2+ anterior chamber cells, vitritis, occlusive retinal vasculitis, and retinal whitening consistent with retinal ischemia. FA revealed extensive retinal arterial and venous filling defects and multifocal hypoperfusion of the choroid; late-phase images showed arterial and venous staining and persistent filling defects with mild optic disc hyperfluorescence (Figure 3, A and B). OCT showed hyperreflectivity of the inner retinal layers, consistent with acute retinal ischemia (Figure 3C). Oral prednisone was initiated; the patient has yet to return for follow-up.
Figure 3.

A 78-year-old man with intraocular inflammation progressing to symptomatic occlusive retinal vasculitis in the left eye 34 days after his first (and only) brolucizumab injection. (A) Early-phase fluorescein angiography revealed diffuse retinal arterial and venous filling defects with extensive ischemia and choroidal hypoperfusion in several small multifocal areas. (B) Late-phase fluorescein angiography images demonstrated persistent arterial and venous filling defects, focal leakage around arterioles and venules, slowly resolving choroidal hypoperfusion, and mild optic nerve hyperfluorescence. (C) Optical coherence tomography showed hyperreflective thickening of the inner retinal layers in the nasal macula, consistent with acute retinal ischemia.
Case 4.
An 87-year-old woman with NVAMD in the right eye and 65 prior anti-VEGF injections (most recently aflibercept) was switched to brolucizumab in an attempt to improve anti-VEGF efficacy. She had a history of type 2 diabetes and hypertension, and she was pseudophakic in both eyes. VA in the fellow eye was 20/200. At the time of her first (and only) brolucizumab in the right eye, VA was 20/70. Twenty-three days later, the patient returned to the clinic with blurry vision. VA was still 20/70, but there were 2+ anterior chamber cells noted; examination was otherwise unremarkable.
The patient was started on topical corticosteroids, but inflammation persisted on 2 follow-up examinations. Fifty-eight days after brolucizumab, the patient noted worsening vision, floaters, and pain. VA had declined to counting fingers at 3 feet, and examination was remarkable for 3+ anterior chamber cells. No vitritis was noted, but there was retinal arterial occlusion/sheathing with extensive macular retinal whitening and a cherry-red spot, consistent with ischemia (Figure 4). One month later (86 days after brolucizumab), VA declined to light perception, anterior chamber inflammation resolved, vitritis was noted, and retinal examination was unchanged.
Figure 4.
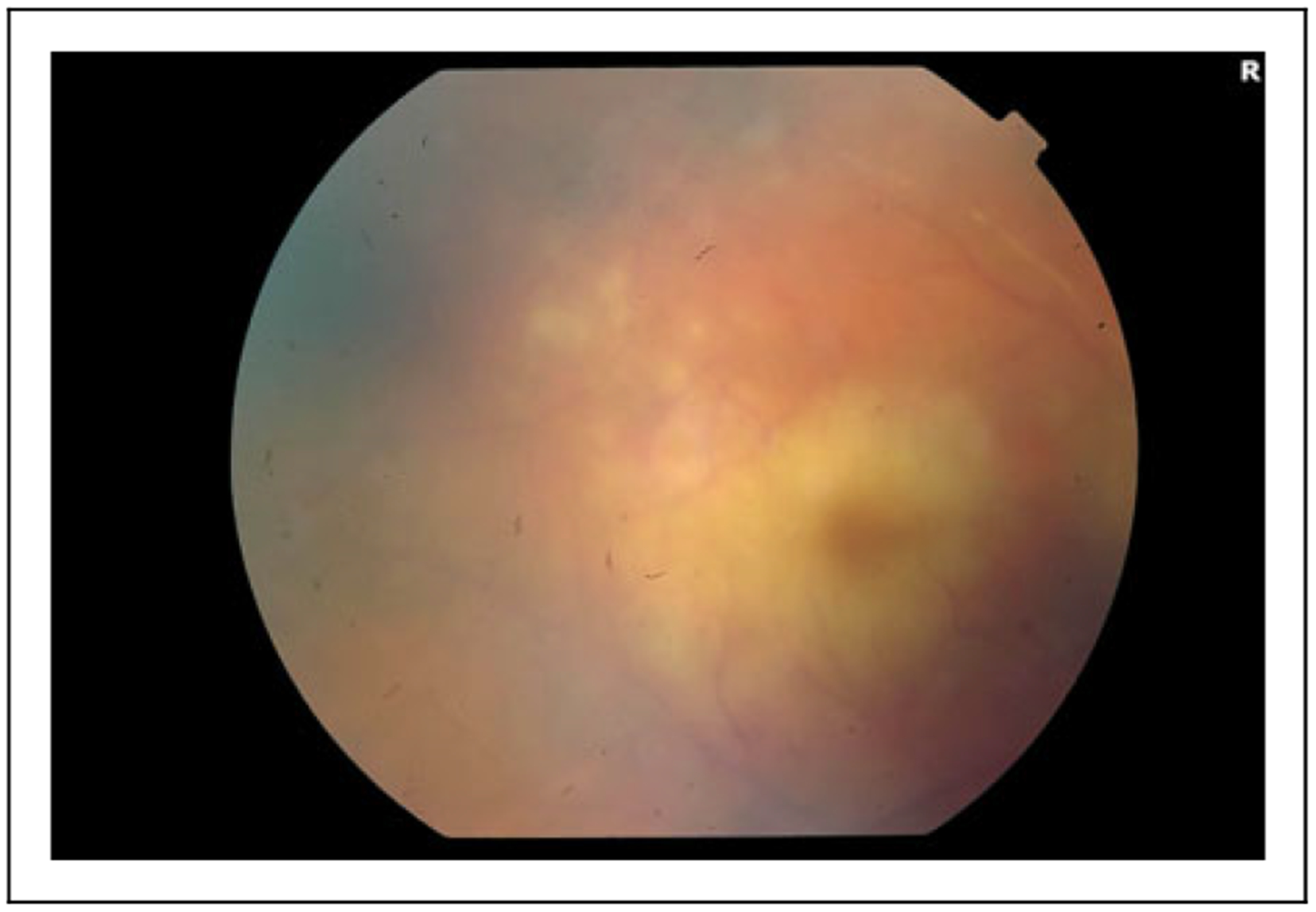
An 87-year-old woman with intraocular inflammation progressing to symptomatic occlusive retinal vasculitis in the right eye, noted 58 days following her only brolucizumab injection. Visualization is hazy with 3+ anterior chamber cells, but retinal arterial occlusion/sheathing is visible along the superotemporal arcade with prominent macular whitening and a cherry-red spot.
Conclusions
As of April 1, 2020, the ASRS collected and analyzed data from 26 eyes of 25 patients with retinal vasculitis occurring after intravitreal brolucizumab for NVAMD. Most eyes had evidence of retinal vascular occlusion and/or retinal ischemia on examination and/or imaging (83%−88%). Most cases (92%) were associated with intraocular inflammation. Common examination findings included visible arterial occlusion/sheathing/boxcarring, venous dilatation and beading, and retinal whitening consistent with ischemia; retinal hemorrhages were sparse if present. There was a predilection for retinal arteries more than veins, although both arteries and veins were affected in 67% of cases. Nearly half (48%) demonstrated multifocal choroidal ischemia on angiography. Although a few eyes in this series were asymptomatic or minimally symptomatic at the most recent follow-up visit, 46% of eyes had a decline of VA of 3 lines or more compared with baseline, 35% of eyes had a decline of VA of 6 lines or more, and 46% of eyes had a VA of 20/200 or less at most recent follow-up.
Etiology
Intraocular inflammation has been associated with bevacizumab (Genentech, Inc), ranibizumab (Genentech, Inc), and aflibercept (Regeneron Pharmaceuticals, Inc) at rates between 0.033% and 2.9% per injection.4 Many cases can be treated successfully with topical or local corticosteroids, although more severe cases are often presumed infectious and treated as such.4,5 There seems to be an underlying rate of intraocular inflammation with all anti-VEGF drugs, and clusters of higher rates of inflammation may also occur.6–11 The mechanism of intraocular inflammation after anti-VEGF remains unknown; suggested mechanisms have included immune response to the drug itself, other protein byproducts within the medication, or differences in pH, whereas mechanisms of inflammation clusters have been attributed to silicone oil residues, silicone/protein aggregates, or endotoxins.8–11
With brolucizumab, in phase 3 clinical trials and according to the FDA label, the rate of intraocular inflammation was higher (>4%) than with previous anti-VEGF drugs (<1%). As of now, the reason for this is unknown. Interestingly, there were high rates of antibrolucizumab antibodies noted during HAWK and HARRIER. Even before drug initiation, 36% to 52% of patients had antibrolucizumab antibodies. After initiation of dosage, antibrolucizumab antibodies were detected in 53% to 67% of patients treated with brolucizumab, and by week 88, 23% to 25% of eyes had induced or boosted levels of antibrolucizumab antibodies.12,13 There was a higher percentage (6%) of patients with intraocular inflammation among patients testing positive for antibrolucizumab antibodies compared with patients without these antibodies (2%). Conversely, neither preexisting nor treatment-emergent antibrolucizumab antibodies seemed to affect the efficacy of the drug, and the clinical significance of these antibodies remains unclear.
In comparison to brolucizumab, clinical trials with ranibizumab and aflibercept have shown 0% to 3% of patients with antidrug antibodies before treatment initiation and 1% to 9% of patients with antidrug antibodies after a 2-year treatment course.14,15 It is possible that the higher rates of preexisting and treatment-emergent antibrolucizumab antibodies may help explain higher rates of inflammation in relation to this drug, but it is unclear what their role is (if any) in cases of intraocular inflammation (including retinal vasculitis) after brolucizumab.
To our knowledge, there have not been any previous reports of inflammation-related retinal vasculitis associated with bevacizumab, ranibizumab, or aflibercept. However, there have been rare reports of retinal artery occlusion (in the absence of intraocular inflammation) following injection of these 3 anti-VEGF medications.16–18 The mechanism of these cases was unclear, but most were attributed to underlying cardiovascular disease, which is common in patients requiring anti-VEGF treatment of diabetic macular edema, retinal vein occlusion with macular edema, or NVAMD.
Some authors postulated that anti-VEGF treatment may have also played a role in retinal vascular occlusion, because anti-VEGF medications have been shown to promote vasoconstriction, decrease blood flow velocity, and increase platelet aggregation.18,19 Indeed, systemic anti-VEGF therapy is known to increase risk of hypertension and arterial thromboembolic events.16–18 It is possible that because of its more potent anti-VEGF effect, brolucizumab may have a high enough anti-VEGF effect to cause retinal arteriolar constriction and occlusive vasculopathy compared with other anti-VEGF agents. Perhaps this effect could explain the presence of vascular occlusion in eyes with no other signs of intraocular inflammation. However, this would not explain the presence of intraocular inflammation in most eyes in this series; the presence of this additional finding suggests an inflammatory or a combined mechanism.
In the present series, the time course and presentation of inflammation in most eyes suggest a delayed immune reaction to the drug or some component of the delivery system. The delay in presentation and clinical findings is not typical for postinjection endophthalmitis, which most commonly appears within the first week after an injection and is more commonly associated with pain, conjunctival injection, hypopyon, and dense vitritis with a minimal view to the retina (although vasculitis can be an examination finding of infectious endophthalmitis in severe cases).20 The appearance of the disease differs from hemorrhagic occlusive retinal vasculitis (HORV), which is another delayed-onset occlusive retinal vasculitis that has been associated with intraocular injection of vancomycin.21
In HORV, the most characteristic examination findings include diffuse retinal hemorrhages (often along the venules) and venous sheathing. Conversely, in occlusive retinal vasculitis after brolucizumab, arterial occlusion and sheathing, sometimes accompanied by angiographic multifocal choroidal ischemia, are prominent features, whereas retinal hemorrhages and venous sheathing are rare. The etiology of HORV remains unclear, but authors have suggested it may be related to a type III (immune complex–mediated) or a type IV (T cell–mediated) hypersensitivity reaction to vancomycin.21,22 The differences in clinical appearances of these 2 diseases suggest different immunologic mechanisms, and it remains unclear what the mechanism is in each of these diseases. If antibrolucizumab antibodies are a key feature in the etiology of inflammation after injection of brolucizumab, perhaps the deposition of immune complexes plays a role in the retinal vascular occlusion seen in these cases.
None of the patients in this series were treatment naive, consistent with the population of patients treated with a newly available drug. Of note, vascular occlusion was also reported in the phase 3 HAWK and HARRIER trials, which included only treatment-naive NVAMD patients.1,13 In our series, occurrence of retinal vasculitis did not appear to be associated with the combination of brolucizumab with any other particular anti-VEGF agent. The condition occurred in eyes that had received any of the 3 other anti-VEGF drugs prior to injection of brolucizumab. In addition, there were 2 eyes that worsened after injection of another anti-VEGF agent in the presence of intraocular inflammation—one had ranibizumab and the other had aflibercept. The contributory role of repeat anti-VEGF treatment in the setting of existing brolucizumab-related inflammation is unclear, but progression after 2 different agents further suggests that the condition was not caused by an interaction between a specific anti-VEGF agent and brolucizumab.
Epidemiology
In this series, retinal vasculitis after brolucizumab affected women more than men. Given the small number of patients, it is unclear whether this discrepancy indicates a true disparity between sexes in this disease, or whether it might be due to chance. The age and race of patients affected were similar to the general NVAMD population in the United States. There was no indication of an association with any ocular disorders, autoimmune diseases, drug allergies, or other medical disorders.
The incidence of retinal vasculitis after brolucizumab remains unclear. Although Novartis estimates that as of March 27, 2020, there had been 70000 vials injected in the United States in 37000 unique patients, the number of postapproval cases is unknown for several reasons (data provided by Novartis via personal communication with P.H. April 3, 2020). First, voluntary reporting of AEs is inherently subject to underreporting, and we are aware of other cases of retinal vasculitis after brolucizumab that have not been reported to the ASRS, including 2 recently published case reports.2,3 We are also aware of other case series being collected, and there is likely some overlap of cases with our series. Second, cases of retinal vasculitis may be mild and difficult to detect without a careful dilated fundus examination and/or angiography. Third, the incidence of this disease may change with repeated injections and/or evolve with further experience with brolucizumab, which had been on the market for less than 6 months at the conclusion of the data collection period for this study.
In the HAWK and HARRIER trials, retinal artery occlusion was reported at a rate of 0.8% (6 of 730 patients) in eyes treated with brolucizumab,1,13 but it is unclear at this time whether those cases represent the same condition as the cases of retinal vasculitis reported in this article. Analysis of completed, ongoing, and future phase 3 and postmarketing trials will be important to better define the incidence.
Study Limitations
The information in this report is limited to data that were voluntarily submitted to the ASRS ReST Committee by the reporting physicians. Follow-up was limited to the termination date of data collection, and some patients had ongoing inflammation and retinal vascular occlusion that may improve with longer follow-up. It is possible that VA results may have also been affected by lack of long-term follow-up. However, in the 8 eyes with follow-up more than 60 days after the most recent brolucizumab treatment, VA results were similar to those in the entire cohort, suggesting that VA outcomes may have been similar even with longer follow-up (see Table 2). Because only 1 patient had ICGA imaging, it is possible that the rate of choroidal involvement was underestimated in this study, and additional cases could have been seen if more patients had ICGA imaging. Without long-term follow-up and with a limited number of cases with a range of severity and treatment approaches, this study was unable to determine optimal treatment modalities.
In addition, at the time of this analysis, brolucizumab had been on the market for less than 6 months. In this series, all cases of retinal vasculitis arose after 1, 2, or 3 brolucizumab injections, but it is unlikely that patients were treated with more than 3 injections in that time frame. Longer-term experience with this drug will be important to understand outcomes and whether vasculitis can arise at any time during a patient’s treatment course or is seen only after the first few injections of brolucizumab.
Recommendations
Although the exact mechanism of these findings remains unclear, the ReST Committee recommends a careful evaluation of the anterior and posterior segment for any signs of active inflammation prior to any brolucizumab injection. Appropriate informed consent should be obtained, and patients should be advised to return for prompt evaluation with changes. Any inflammation following brolucizumab should be followed closely, because occlusive vasculitis has been noted to develop subsequently in a delayed fashion. Widefield angiography is helpful in visualizing the spectrum of vasculopathy in these patients. The data from this series were not sufficient to make any conclusive statements in regard to optimal treatment strategies. In continued treatment of NVAMD in patients with retinal vasculitis after brolucizumab, retreatment with anti-VEGF medication should be considered after intraocular inflammation has resolved completely. Because of the potentially severe nature of the consequences of retinal vasculitis secondary to brolucizumab, caution is advised when considering injection of brolucizumab in monocular patients or when bilateral injections are being contemplated.
Acknowledgments
The ASRS ReST Committee would like to recognize the vigilance and reporting of the following physicians (in alphabetical order), as well as those who prefer to remain anonymous: Mark E. Chittum, Matthew Dombrow, Kori Elkins, K. Bailey Freund, Paul Gallogly, April Harris, Vishak John, Srinivas Sai Kondapalli, Rohit Lakhanpal, Mattew Manry, Sachin Mehta, Patrick Oellers, Marc Peden, Ryan Rich, Jason Paul Ruggiero, Bryan Rutledge, Newman Sund, Elizabeth VernerCole, Mark Walsh, Scott Walter, John Welch, Steve Witkin, and Matthew Wood.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Institutes of Health (Grant EY027691).
Ethical Approval
This study was deemed exempt from institutional review board oversight based on personal communication with the Human Research Protection Program at Allina Health System (Minneapolis, Minnesota).
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Relevant potential conflicts of interest are reported as follows: A.J.W.: none; P.H.: Genentech (consultant, lecture fees) and Allergan (consultant); T.G.M.: none; J.F.A.: Springer SBM LLC (patents/royalties), DORC International B.V. (consultant, lecture fees), Allergan Inc (consultant, lecture fees), Bayer (consultant, lecture fees), Mallinckrodt (consultant), and TOPCON (grant support); K.J.B.: Bausch&Lomb (consultant, lecture fees), Allergan (consultant, lecture fees), Regeneron (consultant, lecture fees), Novartis (consultant, lecture fees), and Genentech (consultant); N.C.: Allergan (consultant), Bayer (consultant), Novartis (consultant); G.G.E.: Novartis (stock), and Regeneron (stock); R.A.G: Genentech (research, speaker, advisory), Regeneron (advisory), Novartis (research, speaker, advisory), Allergan (research, speaker, advisory), Aerie (research), Santen (research), Graybug (research), and NovoNordisk (research); S.J.K.: none; J.P.: none; E.W.S.: none; H.T.: Alimera (stock); R.W.W.: Novartis (research).
Statement of Informed Consent
Because this study did not involve human participants but rather deidentified patient information submitted to the ASRS ReST Committee, informed consent was not obtained.
References
- 1.Dugel PU, Koh A, Ogura Y, et al. ; HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1): 72–84. doi: 10.1016/j.ophtha.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 2.Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi: 10.1016/j.ajoc.2020.100680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A, Chea S, Matsumiya W, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;18: 100687. doi: 10.1016/j.ajoc.2020.100687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal S, Joshi M, Christoforidis JB. Vitreous inflammation associated with intravitreal anti-VEGF pharmacotherapy. Mediators Inflamm. 2013;2013:943409. doi: 10.1155/2013/943409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson D, Sharma S. Ocular and systemic safety of bevacizumab and ranibizumab in patients with neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2013;24(3):205–212. doi: 10.1097/ICU.0b013e32835f8ec0 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg RA, Shah CP, Wiegand TW, Heier JS. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol. 2014; 158(4):733–737.e1. doi: 10.1016/j.ajo.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 7.Hahn P, Chung MM, Flynn HW Jr, et al. Postmarketing analysis of aflibercept-related sterile intraocular inflammation. JAMA Ophthalmol. 2015;133(4):421–426. doi: 10.1001/jamaophthalmol.2014.5650 [DOI] [PubMed] [Google Scholar]
- 8.Greenberg JP, Belin P, Butler J, et al. ; Aflibercept Sterile Inflammation Research Group. Aflibercept-related sterile intraocular inflammation outcomes. Ophthalmol Retina. 2019;3(9):753–759. doi: 10.1016/j.oret.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 9.Georgopoulos M, Polak K, Prager F, Prünte C, Schmidt-Erfurth U. Characteristics of severe intraocular inflammation following intravitreal injection of bevacizumab (Avastin). Br J Ophthalmol. 2009;93(4):457–462. doi: 10.1136/bjo.2008.138479 [DOI] [PubMed] [Google Scholar]
- 10.Ness T, Feltgen N, Agostini H, Bohringer D, Lubrich B. Toxic vitreitis outbreak after intravitreal injection. Retina. 2010;30(2): 332–338. doi: 10.1097/iae.0b013e3181baf691 [DOI] [PubMed] [Google Scholar]
- 11.Melo GB, Figueira ACM, Batista FAH, et al. Inflammatory reaction after aflibercept intravitreal injections associated with silicone oil droplets released from syringes: a case-control study. Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):288–294. doi: 10.3928/23258160-20190503-05 [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. Center for Drug Evaluation and Research. Published September 25, 2019. Accessed June 11, 2020 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125Orig1s000SumR.pdf
- 13.US Food and Drug Administration. Published October 2019. Accessed June 11, 2020 https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761125s000lbl.pdf
- 14.US Food and Drug Administration. Center for Drug Evaluation and Research. Published April 15, 2017. Accessed June 11, 2020 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/125156Orig1s114.pdf
- 15.Food US and Administration Drug. Center for Drug Evaluation and Research. Published March 30, 2012. Accessed June 11, 2020 https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/125418Orig1s000ClinPharmR.pdf
- 16.Mansour AM, Bynoe LA, Welch JC, et al. Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmol. 2010;88(7): 730–735. doi: 10.1111/j.1755-3768.2009.01535.x [DOI] [PubMed] [Google Scholar]
- 17.Chuang LH, Wang NK, Chen YP, Wu WC, Lai CC. Mature vessel occlusion after anti-VEGF treatment in a retinal arteriovenous malformation. BMC Ophthalmol. 2013;13:60. doi: 10.1186/1471-2415-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Borkar D, Obeid A, Hsu J, Ho AC, Garg SJ. Incidence of retinal artery occlusion following intravitreal antivascular endothelial growth factor injections. Acta Ophthalmol. 2019;97(6): e938–e939. doi: 10.1111/aos.14058 [DOI] [PubMed] [Google Scholar]
- 19.Sacu S, Pemp B, Weigert G, et al. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2011;52(6):3046–3050. doi: 10.1167/iovs.10-5842 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz SG, Flynn HW Jr, Emerson GG, et al. Distinguishing between infectious endophthalmitis and noninfectious inflammation following intravitreal anti-VEGF injection. J Vitreoret Dis. 2019;3(1):42–44. doi: 10.1177/2474126418806832 [DOI] [Google Scholar]
- 21.Witkin AJ, Chang DF, Jumper JM, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology. 2017;124(5):583–595. doi: 10.1016/j.ophtha.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 22.Todorich B, Faia LJ, Thanos A, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: a clinical-pathophysiological analysis. Am J Ophthalmol. 2018;188: 131–140. doi: 10.1016/j.ajo.2018.01.030 [DOI] [PubMed] [Google Scholar]


