Fig 1. Patient enrollment and sample collection.
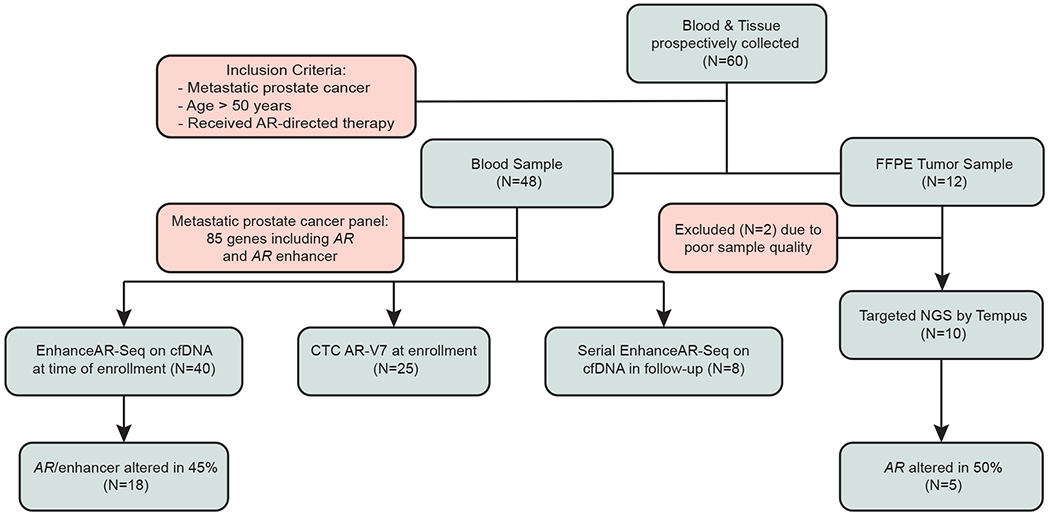
Patients with biopsy-proven metastatic prostate cancer treated with AR-directed therapy were enrolled onto the study and samples collected for tissue, cell-free DNA and CTC analyses. AR, androgen receptor; CTC, circulating tumor cell; EnhanceAR-Seq, Enhancer and neighboring loci of Androgen Receptor Sequencing; cfDNA, cell-free DNA; FFPE, formalin-fixed paraffin-embedded; NGS, next-generation sequencing.
