Fig 4. Serial timepoint liquid biopsy analyses of patients on AR-directed treatment.
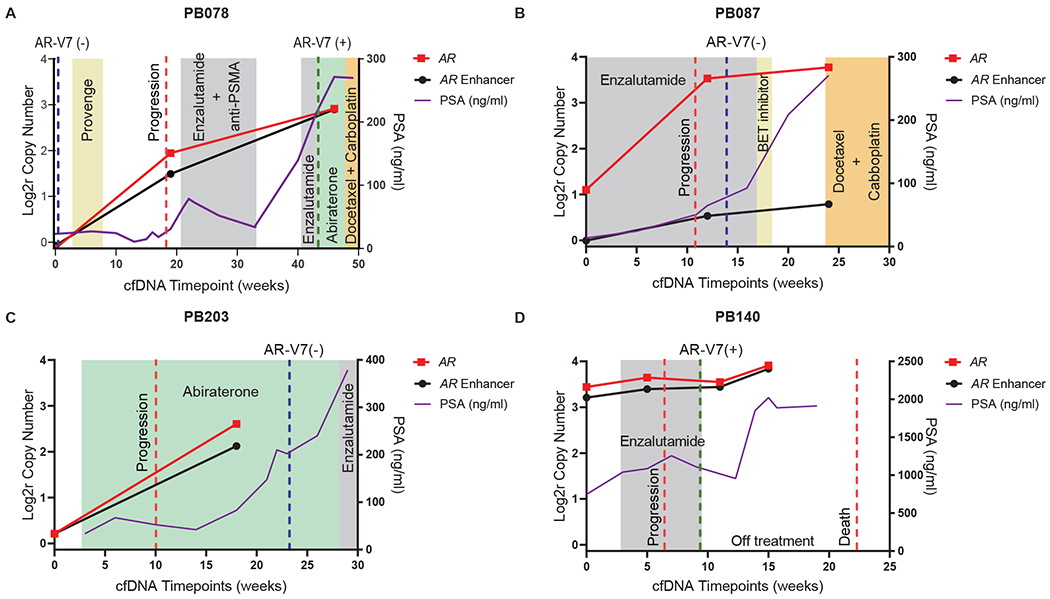
(A) The patient was negative for CTC AR-V7 and cell-free DNA AR/enhancer alteration at the time of enrollment. At week 19, shortly before receiving enzalutamide and anti-PSMA, he tested positive for amplifications in AR and its enhancer in cfDNA. The patient initially responded, then after a treatment break rapidly progressed on both enzalutamide and abiraterone. Repeat testing at this final timepoint (~45 weeks post-enrollment) was positive with further amplification observed in AR and its enhancer in cfDNA and AR-V7 detected in CTCs. (B-D) Clinical vignettes of three more mCRPC patients with serial cfDNA collected over time with at least one timepoint occurring during AR-directed therapy. AR and AR enhancer copy number ratios in cfDNA are shown over time in log2 space, and PSA concentrations in blood are shown in ng/mL. Treatments are indicated in colored boxes, time of progression or death as dashed red lines, and AR-V7 test results as dashed green lines (if positive) or blue lines (if negative). Weeks since study enrollment are shown on the X-axis. cfDNA, cell-free DNA; CTC, circulating tumor cell; AR, androgen receptor; Log2r, logarithm base 2 ratio; PSMA, prostate-specific membrane antigen; PSA, prostate-specific antigen; BET, bromodomain and extraterminal domain; mCRPC, metastatic castration resistant prostate cancer.
