Figure 5. Representative peptographs of extracts of ruptured vs. stable human plaque segments.
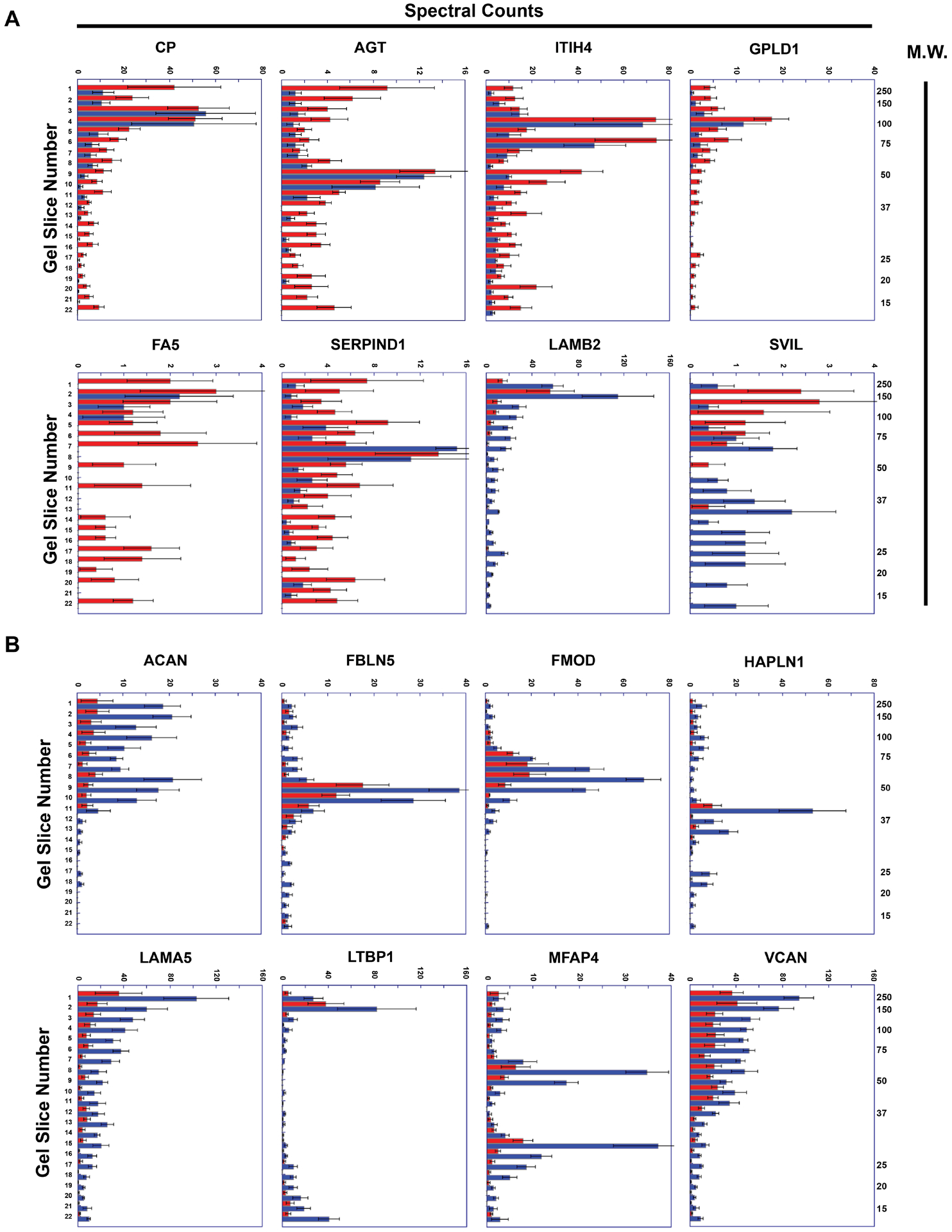
(A and B) Extracts of ruptured (red) and adjacent stable (blue) segments of 5 human carotid plaques were analyzed using the PROTOMAP protocol. The extracts were subjected to SDS-PAGE and the gels were cut into 22 slices, each corresponding to a molecular-weight range. After in-gel trypsin digestion, peptides were extracted, identified by tandem mass spectrometry, and spectral counts were aggregated over all 22 slices. (A) Proteins with differential abundance of lower-molecular-weight peptides in extracts of ruptured vs. stable segments: ceruloplasmin (CP), angiotensinogen (AGT), inter-alpha-trypsin inhibitor heavy chain (ITIH4), phosphatidylinositol-glycan-specific phospholipase D (GPLD1), coagulation factor V (FA5), heparin cofactor 2 (SERPIND1), laminin subunit beta-2 (LAMB2), and supervillin (SVIL). (B) ECM proteins that are significantly less abundant in extracts of ruptured vs. stable human plaque segments: aggrecan core protein (ACAN), fibulin-5 (FBLN5), fibromodulin (FMOD), hyaluronan and proteoglycan link protein 1 (HAPLN1), laminin subunit alpha-5 (LAMA5), microfibril-associated glycoprotein 4 (MFAP4), latent-transforming growth factor beta-binding protein 1, and versican (VCAN). (A and B) Horizontal bars in each peptograph portray the total spectral counts for protein-specific peptides in each of the 22 gel slices (mean ± SEM; n = 5). Gel-slice number is on the leftward y-axis; molecular weight of the gel slices (in kiloDaltons) is on the rightward y-axis.
