Figure 2.
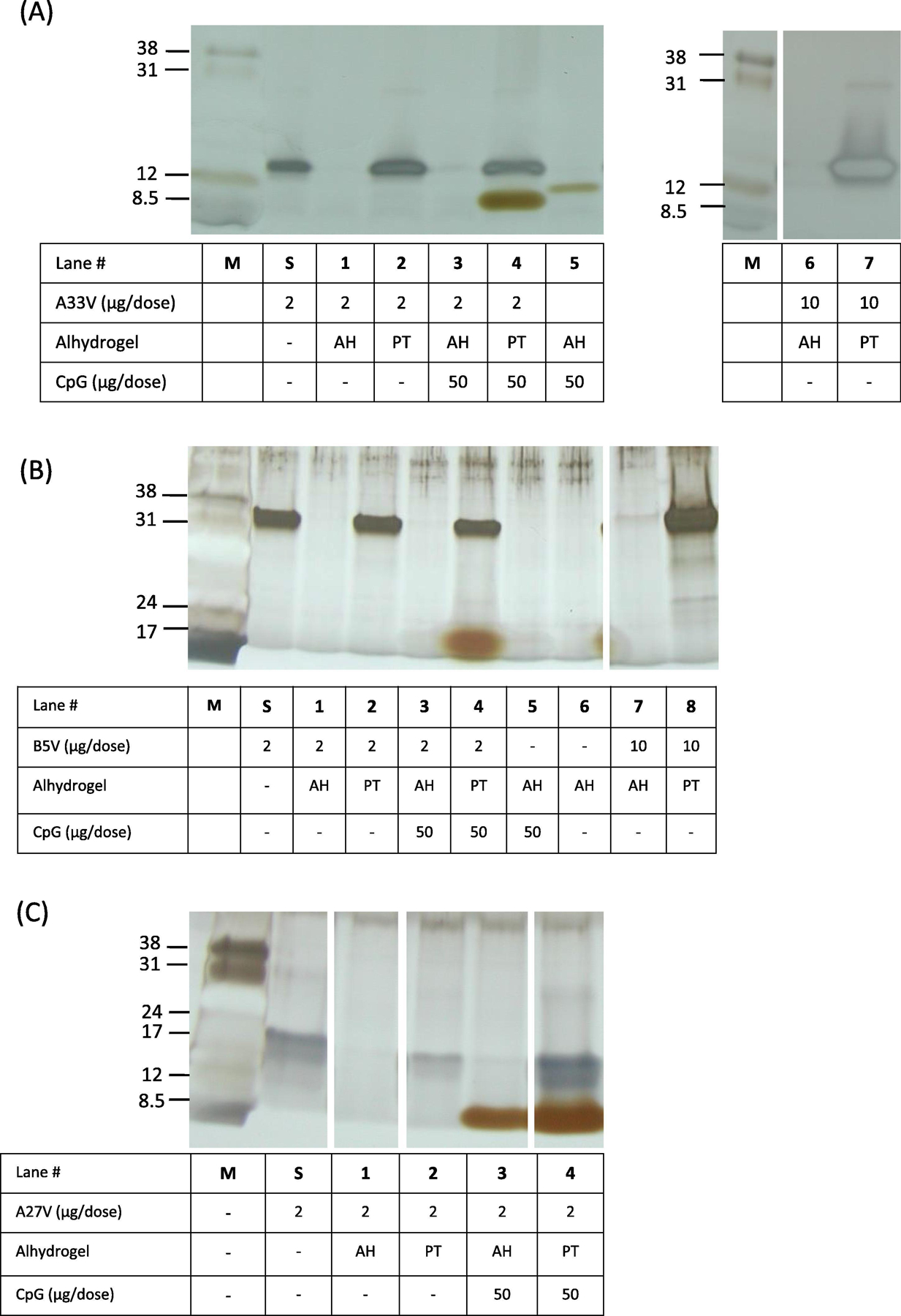
Characterization of vaccine formulations by silver-staining of SDS-PAGE. After vaccine formulations were made, the tube was centrifuged to pellet the solid aluminum hydroxide and the supernatants (5 μl) from each vaccine formulation were processed and loaded in each lane. Lane “M” is low-range molecular weight markers (Amersham) with the indicated apparent molecular weights in kDa. Lanes “S” is the starting amount of protein at a concentration of 2 μg/50 μl of A33V, B5V, A27 in histidine buffer. A. A33V formulations. PT = 40 mM KH2PO4. B. B5V formulations. PT = 40 mM KH2PO4. C. A27V formulations. PT = 100 mM KH2PO4. Note, the band in panel A, lane 5, is spill over from unrelated adjacent lane to the right of lane 5. AH, Alhydrogel; PT, phosphate treated Alhydrogel at the indicated concentration.
