Figure 1. Transcriptional regulation determines normal B cell development.
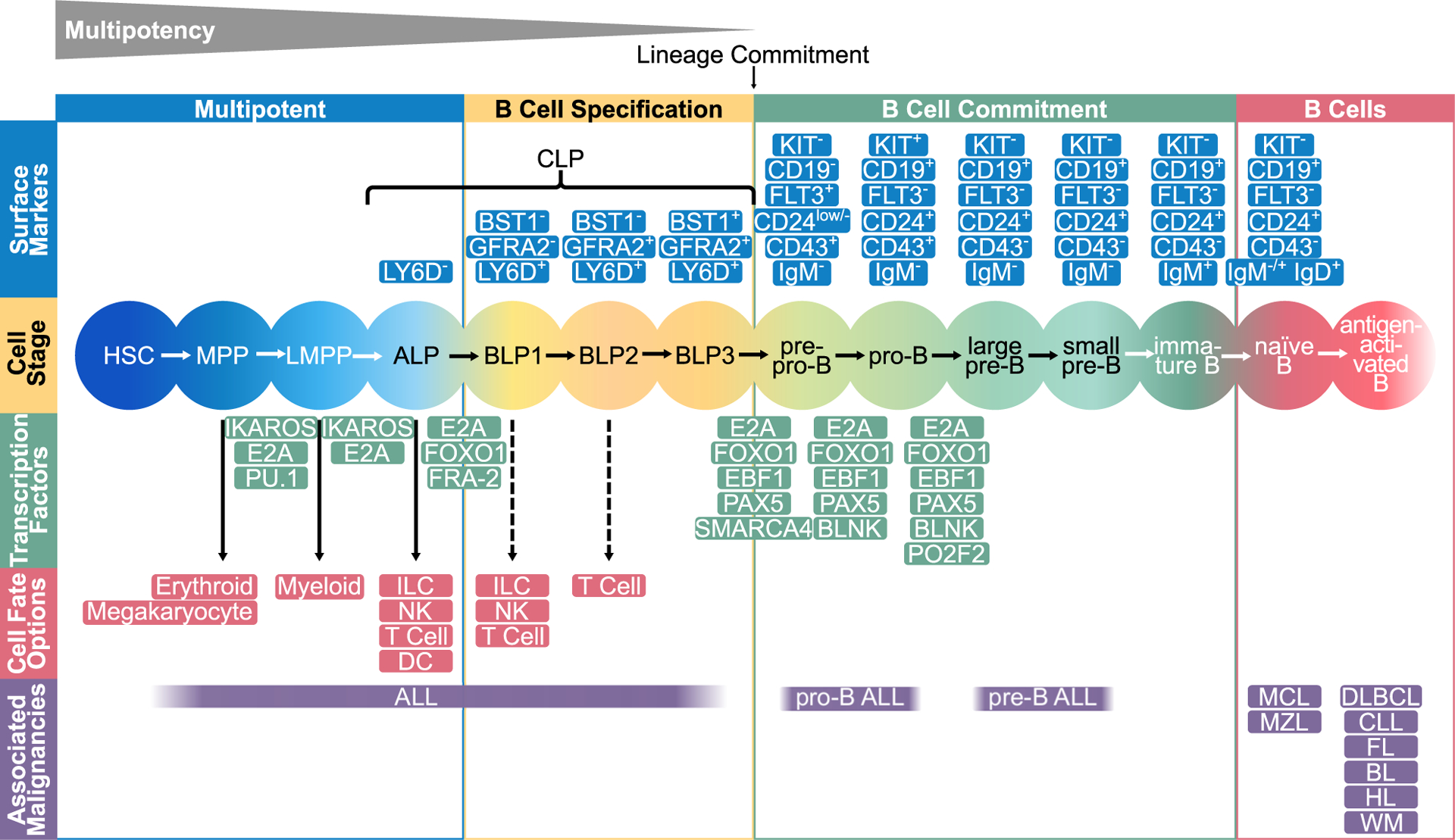
A global network of regulatory circuits and epigenetic factors drives differentiation of hematopoietic stem cells (HSC) through B cell lymphopoiesis. The first stages of B cell development are marked by multipotent cell types that can also develop in very different hematopoietic cell types. Potential other developmental paths are given in red boxes. The most important transcription factors that drive the differentiation from one progenitor to another are presented in green between the developmental stages. Surface markers are given in blue and hematopoietic malignancies associated with the cell stage in purple. The multipotent cells are succeeded by B cell-biased lymphoid progenitors (BLP) that are already primed towards becoming B cells but still have other lymphoid options. The BLP stages are characterized by the expression of the surface proteins BST1 and GFRA2. After full lineage commitment, BLP3 develop into pro-B cells. The transcription factors E2A, EBF1, and PAX5, in combination with several epigenetic factors, play central roles in the B cell fate decision. MPP, multipotent progenitors; LMPP, lymphoid-primed MPP; CLP, common lymphoid progenitor; ALP, all-lymphoid progenitor; ILC, innate lymphoid cell; NK, natural killer cell; DC, dentritic cell; ALL, acute lymphoblastic leukemia; MCL, mantle cell lymphoma; MZL marginal zone B cell lymphoma; DLBCL, diffuse large B cell lymphoma; CLL, chronic lymphocytic leukemia; FL, follicular center cell lymphoma; BL, Burkitt’s lymphoma; HL, Hodgkin’s lymphoma; WM, Waldenström’s macroglobulinemia.
