Figure 1. PARP1 Domain Structure.
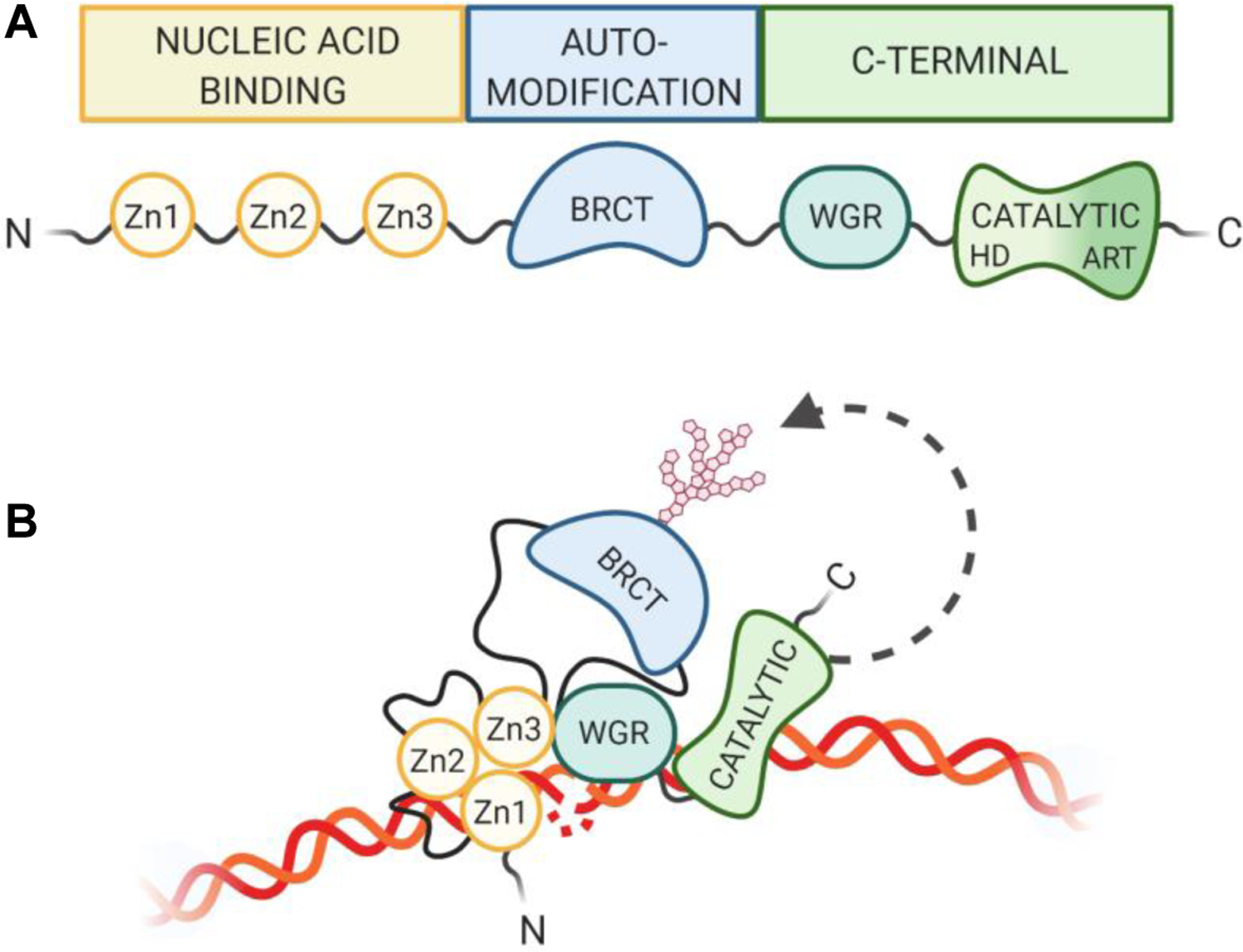
(A) PARP1 is composed of 3 main regions, consisting of 6 independently folded domains connected by flexible linker regions. The N-terminal nucleic acid binding region contains three zinc finger domains (Zn1, Zn2, Zn3). The BRCT-containing automodification domain lies in the PARP1 interior. The C-terminal region contains an additional nucleic acid binding motif (WGR) as well as the catalytic domain, which is made up of an alpha helical subdomain and the highly conserved PAR signature subdomain. (B) PARP1 adopts a collapsed conformation for variable substrate recognition during activation (Langelier, Zandarashvili, Aguiar, Black, & Pascal, 2018; Rudolph, Mahadevan, Dyer, & Luger, 2018).
