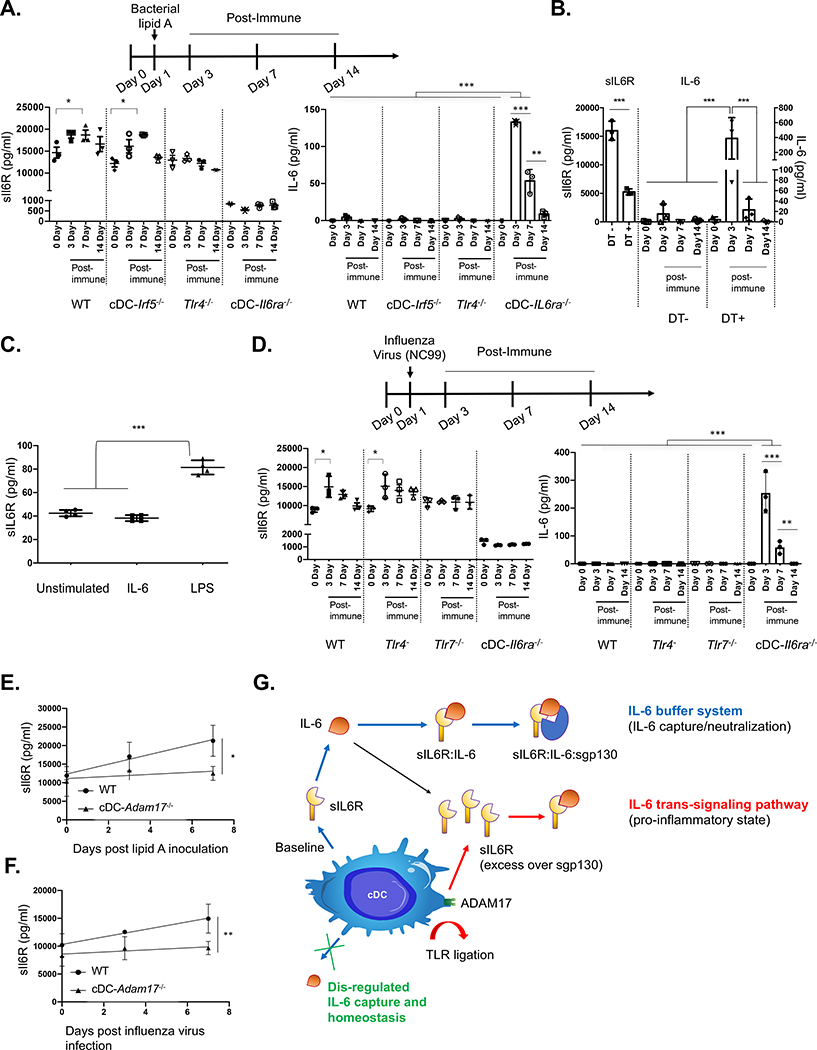
Figure 4. cDCs regulate the systemic sIL-6R set point and induction of release, regulating the pharmacokinetics of free IL-6 generated in response to diverse immune challenges.
(A) Animals were injected intravenously with bacterial lipid A to mimic sepsis and promote IL-6 release. Serum concentrations of sIL-6R and IL-6 were measured in WT, cDC− Irf5−/−, Tlr4−/−, and cDC-IL6ra−/− (mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, ANOVA with Tukey’s test; n = 3 per group).
(B) sIL-6R and IL-6 serum concentrations during the same time course in Zbtb46-DTR chimeras receiving and not receiving DT (mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, ANOVA with Tukey’s test; n = 3 per group).
(C) BMDCs were cultured and exposed to recombinant IL-6 or bacterial LPS, and sIL-6R was measured in the supernatant (mean SD; ****p < 0.0001, ANOVA with Tukey’s test).
(D) Animals were infected with a sublethal dose of NC99 influenza virus, and serum sIL-6R and IL-6 concentrations were measured in WT, Tlr4−/−, Tlr7−/−, and cDC-IL6ra−/− (mean ± SD; *p < 0.05, ***p < 0.001, ANOVA with Tukey’s test; n = 3 per group). In bacterial and viral challenge models, the defect in IL-6 capture within cDC-IL6ra−/− was rescued by restoring sIL-6R using AAV gene delivery (Figures S4A and S4B). Restoration of sIL-6R also reduced the morbidity rate after lethal challenge with influenza virus (Figure S4C).
(E and F) cDC-Adam17−/− had no defect in the sIL-6R set point but significantly blunted sIL-6R release following stimulation with bacterial lipid A (E) or infection with influenza virus (F) (mean ± SD; *p < 0.04, **p < 0.01, F test for slope comparison; n = 5 per group).
(G) Regulating free IL-6 and IL-6 trans signaling via cDC-derived sIL-6R.
Please also see Figure S4.