Fig 3. Computational modeling of MAP2 pS1782 alters predicted structure and reduces microtubule binding.
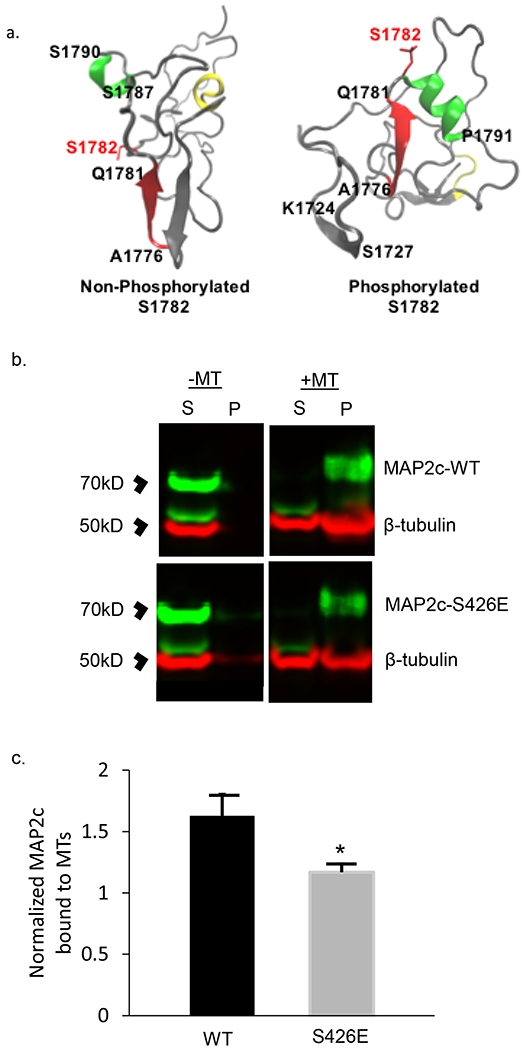
(a) Snapshot of the C-terminal domain of MAP2. Amino acids A1776 to Q1781 form an extended β strand (red β strand) that is occluded by an opposing β strand. In much of the non-phosphorylated ensemble, there is found to be helical propensity in or near regions S1759 to R1765 (yellow) which is not found in the phosphorylated ensembles. In pMAP2 (pS1782) the β strand at A1776 to Q1781 is no longer occluded by another β strand; this is predicted to reduce microtubule binding and enhance interactions with other proteins. The effect of phosphorylation is recapitulated by phosphomimetic mutation S1782E (data not shown). (b) Reduced MT-binding in MAP2c with the phosphomimetic mutation S426E, homologous to S1782 in full length MAP2b relative to MAP2c-WT. 30μL of cell lysate from transiently transfected HEK293 cells was subjected to in vitro MT-binding assay and supernatant (S) and pellet (P) fractions were subjected to SDS-PAGE. (c) Densitometric analysis of gels shows decreased MT binding in the S426E mutant compared to WT. Data shown are from 3 independent experiments, ± SEM. *p < 0.05
